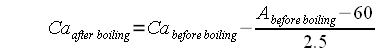Difference between revisions of "Mash pH control"
(→Acids) |
(→Other factors) |
||
| Line 161: | Line 161: | ||
==Other factors== | ==Other factors== | ||
| − | The mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates | + | The mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates <ref name="Narziss_Back">. |
| + | |||
| + | A rest between 30 and 40 C (90-110 F), known as acid rest, promotes phytase activity which releases more phosphates into the mash. While this is said to acidify the mash I have not observed a significant mash pH decrease though the use of this rest. | ||
|- | |- | ||
Revision as of 04:24, 24 February 2011
|
This is the 3rd and last article in a series of articles intended to educate the interested brewer about pH in brewing. Part 1, An Overview of pH, showed the basic principles behind pH and how it can be measured, part 2, How pH affects brewing, illustrated the effects pH has on various brewing processes and which pH ranges are considered optimal. This article will be the most practical. It shows how malt and water settle at a pH and how this pH can be affected through changes in water composition, malt bill and mash additions. For the interested brewer, it also goes behind the scenes and explains the chemical processes that are at work. A number of water treatment and brewing water building options are explained; starting with the basic and ending with more sophisticated ones.
ContentsWater, malt and mash pH | |
|
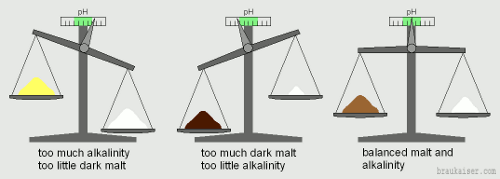
Water and malt are both pH buffers, that means they have their own pH and a desire to resist pH changes. When they are brought together at dough-in, they will settle at a pH which will be the mash pH. Which pH they'll settle at depends on how strong either of the components (water and malt) pull pH to their respective sides. In this "tug of war" malt is the acidic one, it wants to lower pH, and water tends to be the alkaline component, it wants to raise pH. As we have seen in Enzymatic Activity the mash pH range that works for brewing is fairly wide. 5.0 – 6.0 will work with most enzymatically strong malts and 5.3-5.5 is considered optimal. This wide range of possible mash pH values is the reason why most brewers don't have to worry about mash pH and water chemistry at the beginning of their home brewing career. As we will see later, average water and grist compositions generally found in (home) brewing are likely to result in a mash pH in that range. Only at the extremes will brewers experience problems in mash conversion and off flavors that arise from incorrect mash pH levels and spark the brewer's interest in that rather technical topic. But even if you don't experience off flavors or mash problems from incorrect mash pH, there is likely a point in your brewing career where the beers are good and very enjoyable but you want to take them to excellent. Controlling your mash pH and moving it into a more optimal range has the potential to do that for you. The effects that pH has in brewing were elaborated in How pH affects brewing and they start with a proper mash pH. A correct mash pH is all about matching the right water with the right grist and possibly the addition of some acid. Since we brewers generally start with a style of beer or recipe in mind, the grist composition is known and we want to know what kind of water modifications are need to get the desired mash pH. In a way this is like balancing a scale (Figure 1). On the left is the malt and on the right is the water. But we are not interested in their actual weight. Instead we want to know how acidic is the malt is and how alkaline is the water. To determine how acidic malt is, I ran experiments were I tested the distilled water mash pH of some base malts and the acidity of various specialty malts. And what I found was that darker malts are generally more acidic than lighter colored malts. While there were a few exceptions to that rule, which we will examine later, we will work with that for now: The darker malts in the grain bill, the more acidic the grist is. That acidity of the malt is counteracted by the alkalinity of the water. Water alkalinity measures the waters ability to neutralize acids and thus resist a change in pH. In fact, it is measured by adding a strong acid to a sample of water until it reaches a pH of 4.3 [1]. The amount of acid needed determines how alkaline the water is. The more acid that is needed, the more alkaline the water is. When grist and water are mixed the alkalinity of the water will neutralize some of the malt’s acidity. Neutralization happens when an acid and a base are brought together. The result is a salt and water and a pH that lies between the pH of the acid and the pH of the base. The acidity of the grist is best thought of as its mash pH with distilled water. Alkalinity present in the water or from the addition of alkaline salts pulls that pH higher (more alkaline) while the addition of acid, calcium or magnesium lowers that pH (more acidic). If, for example, the grist has low acidity because it contains only lightly colored malts and the water has a lot of alkalinity, the mash pH will be higher than desired. If the opposite is true, lots of malt acidity from dark grains and low alkalinity water, the mash pH will be lower than desired. If malt acidity and water alkalinity are balanced, the pH will be in the desired range. |
|
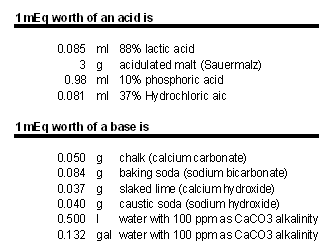
Mash as a pH bufferGrist, water, acid and salt interactions within the mash are best thought of as the addition of acids or bases to a strong buffer which is largely formed by phosphates, proteins and amino acids present in the malt. Dealing with a buffered system means that the amount of acid or base needed to change pH depends on the amount of buffer (in this case weight of the grist) and the desired pH change. The amount of acid it takes to lower or raise the mash pH of 1 kg of grist by 0.1 pH units is about 3-5 mEq. A simple Model for pH Buffers shows how we can visualize pH buffers as differently shaped vertical tubes. mEq (milliequivelent) or Eq (1 mEq = 0.001 Eq) is a unit commonly used to express the amount of acid or bases. Rather than giving the specific amount of acid or base needed it specifies the number of hydrogen ions that are added (acid) or absorbed (base). To convert Eq to an actual amount of acid/base its molar weight, number of hydrogen ions donated/absorbed and its concentration needs to be known. Lactic acid for example has a molar weight of 90 g/mol. Above a pH of 5 almost all its molecules donate 1 hydrogen ion. This means 90g 100% lactic acid contribute about 1 Eq acid. The lactic acid we commonly use has a concentration of 88% by weight and 1 Eq of that weighs 102 g. 1 mEq weighs 0.102 g. Calcium carbonate (chalk), on the other hand, has a molar weight of 100 g/mol but can absorb 2 hydrogen ions. To get 1 Eq worth of acid neutralizing power 50g chalk are needed. In brewing that neutralizing power will be lower due to the acidifying effect of the calcium when it reacts with the malt’s phosphates. But more about this later. Table 1 on the right shows the amounts of a few acids and bases that provide 1 mEq of neutralizing power. The buffer capacity of the malt, which affects the amount of acid or base needed to change mash pH, does to some extend depend on the malting process as well as the mashing process. This may lead to differences how the mash responds to pH correction efforts and makes exact mash pH predictions difficult. |
|
Factors affecting mash pHThe following is a more detailed discussion of what affects mash pH in brewing. |
|
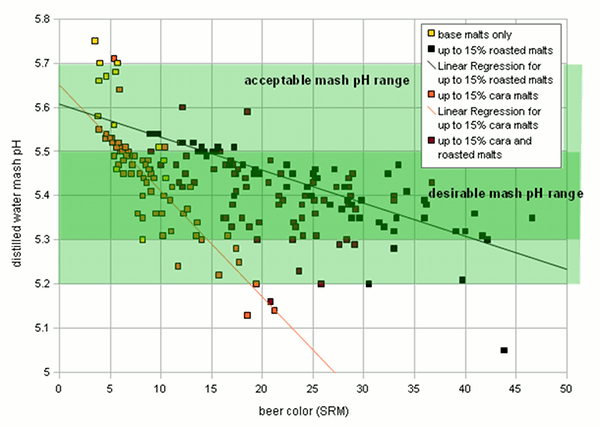
Grist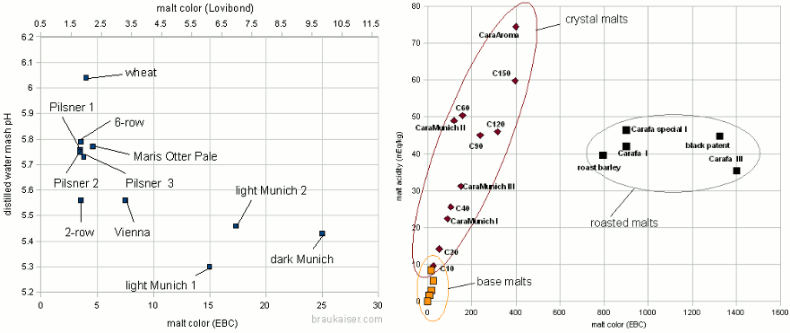 Figure 2 - On the left: the mash pH of mashes with various base when mashed with distilled water. The 2-row that falls out of line for the pale malts is Rahr 2-row. On the right: The titratable acidity of various specialty malts plotted over their color. The test mashes made from these malts were titrated to a pH of 5.7 Very lightly kilned malts have a natural pH between 5.7 and 5.8. Wheat malts may even be higher than that. Once malts are kilned more intensely to produce darker base malts, the distilled water pH drops due to the formation of acidic melanoidens. This is shown in Figure 2 which plots the color and distilled water mash pH for a number of different base malt samples. There is a loose correlation between malt color and mash pH: "The darker the malt the lower the mash pH". Because of this loose correlation the prediction of the malts mash pH based on its color is difficult in particular for darker malts. Specialty malts like crystal and roasted malts are kilned to even darker colors than base malts. While they may constitute a much smaller portion of the grist they tend contribute a large portion of the grist’s color and with it acidity. The latter lowers the grist pH below the pH of the base malts used. The acidity of various specialty malts is plotted over their color in Figure 2. Crystal type malts adhere fairly strongly to the "the darker the more acidic" rule. Roasted malts, on the other hand, showed a fairly constant acidity regardless of their color. This is assumed to be a result production process differences between crystal and roasted malts. The color of crystal malts is created while the malt is still wet which allows for the creation of more acidic compounds while the color of roasted malts is created after they have already been dried. Though that creates a stronger color it creates less acidity[2] For us brewers this means that grists containing large amounts of roasted malts are likely less acidic than grists containing large amounts of dark crystal malts even though the beers made with roasted malts might be darker in color. | |
|
To get a better understanding what crystal and roasted malts do to the acidity of the grist I randomly generated 210 different grist compositions using the malts I analyzed and the following constraints:
The beer color for all these beers was calculated and mash pH was estimated based on the malt data available. The mash pH was then plotted over the beer color and is shown in Figure 3. It is apparent that the majority of the beers falls into the desired mash pH range if the water has no residual alkalinity (What residual alkalinity is will be explained further down in this article). If the was has moderate residual alkalinity the mash pH will be slightly but not dramatically higher. This is the reason why most average beers can be brewed well with average water. Only at the extremes would be mash pH become a problem. Another observation in this figure is that there is a somewhat linear relationship between beer color and mash pH. The slope of that line is steeper for beers that get all or most of their color from crystal malts and flatter for beers that get their color from roasted malts. This relationship can be used to predict grist acidity from the beer color and the percentage of color that is coming from roasted malt. Eventually this can be used to predict mash pH and such an algorithm is described in Beer color, alkalinity and mash pH and has been implemented in the Kaiser_water_calculator.xls. |
|
Water mineralsThe minerals listed here may either come from the base water, from salts added to the water before dough-in or salts added to the mash. Experiments have shown that the source of the minerals does not make much of a difference in their effect on mash pH. What makes a difference though, is the amount of minerals that are brought into the mash. This amount depends on both mash thickness (how much water per unit of grain) and the mineral concentration in the water. In other words, water in a thin mash will be able to have larger effect on mash pH compared to the same water water in a thick mash since in the latter case there will be less water, and with it less minerals, per grain. This being said, brewers should not conclude that thick mashes provide a practical means of dealing with high alkalinity water just because the alkalinity won’t be able to move the mash pH as much. Any water that is not used in the mash is used during the sparge where it is able to adversely affect pH in both the grain bed and boil kettle. This section focuses only on the water minerals which have an effect on pH. A more complete discussion of brewing water composition can be found in How to read a water report. Bicarbonate and AlkalinityAs mentioned in the beginning, alkalinity is the water’s ability to neutralize acids and the ions involved with that neutralization are the bicarbonate (HCO3-) and carbonate ions (CO32-). More info about carbonate chemistry can be found in About chalk and the carbonate system When brought in contact with the more acidic malt the bicarbonate and carbonate ions absorb hydrogen ions which results in a pH rise. The extent of this pH increase largely depends on the amount of malt, amount of water and the bicarbonate and carbonate concentration in that water. The latter is expressed by the water’s alkalinity. The more bicarbonate and carbonate ions there are per unit of malt the more the pH will rise. When bicarbonate or carbonate neutralizes an hydrogen ion it forms carbon dioxide. While bicarbonate is able to neutralize one hydrogen ion carbonate can neutralize two:
Due to the poor solubility of calcium carbonate and the presence of calcium in most waters, appreciable amounts of carbonate are rarely found in brewing water. As a result the bicarbonate concentration in water can be calculated from the alkalinity of the water and vice versa. Carbonate ions will be present when chalk is added to the water or the mash. The addition of chalk, however, will be discussed later. Calcium and MagnesiumBrewing water also contains calcium and magnesium ions. These ions are able to react with phosphates from the malt to form insoluble phosphate salts which precipitate. At mash pH values between 5 and 6 most of the phosphate is available as HPO42-. The reaction with calcium liberates hydrogen ions which react acidic and lower the pH of the mash [3]:
Magnesium shows a similar but less effective reaction with phosphates which is why it has only half the pH lowering power of calcium. Malt contains about 1 % of phosphate by weight[4] . About 80% of that end up in the wort. This amount is far greater than the calcium or magnesium that is brought in with the brewing liquor which makes calcium and magnesium the limiting factor. As early as 1914 did the German brewing scientist Windish show that water ions have an effect on the mash pH. Kolbach, another German brewing scientist, later showed that it takes about 1.75 calcium ions and twice as many magnesium ions to produce one hydrogen ion. (1.75 calcium ions equal 3.5 calcium equivalents) My own research on this subject reported slightly lower numbers: 1.3-1.5 Calcium ions and 2.4-2.8 magnesium ions [Troester]. It should be noted that Kolbachs work was primarily targeted at the cast out wort pH and not so much mash pH. There is however a close correlation between the two. Residual AlkalinityThe acidic reaction of calcium and magnesium counteracts the alkaline reaction of the water’s alkalinity, which prompted Kolbach to define the residual alkalinity as the alkalinity that remains after the calcium and magnesium reactions have been considered. Based on his work the following formula for residual alkalinity RA has been established in the brewing world [5] Where:
The residual alkalinity is a property of the brewing water and allows brewers to estimate a water’s effect on the mash pH. If only alkalinity and general hardness (GH) are known the residual alkalinity can be estimated as: This makes the assumption that about 30% of the water hardness comes from magnesium and the remaining 70% come from calcium, which tends to be the average split between calcium and magnesium hardness in typical waters (see Estimating Residual Alkalinity). Water with a residual alkalinity of 0 gives about the same mash pH as distilled water while water with a RA greater than 0 yields a higher mash pH. If the water’s alkalinity is low but its calcium and magnesium levels are high the residual alkalinity can also be less than 0. In this case the use of that water will yield a lower mash pH. It should be noted that Kolbach's work focused on the pH of the knock-out wort and not mash pH. Wile there is a close relation his finding that a residual alkalinity change of 10 dH (degrees German hardness, about 171 ppm as CaCO3) caused a 0.3 pH change in pH applies to a 12 Plato cast out wort and not the mash pH. The change in mash pH will be less and also depends on mash thickness. AcidsWe may also add acids to the water or mash to lower mash pH below the pH that is established by water and malt alone. This is in particular necessary for lighter beers where the grist itself is not acidic enough to establish the correct pH. Acids donate hydrogen ions which neutralize the alkalinity of the water. Once all the water's alkalinity is consumed the surplus of hydrogen ions counts towards lowering the mash pH. Other factorsThe mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates Cite error: Closing The final calcium level can be calculated with this formula: Where
If the resulting Calcium concentration is below the recommended minimum of 50 mg/l gypsum or calcium chloride should be added to boost the initial calcium content before the water is boiled. In addition to that the addition of a small amount of chalk facilitates this precipitation by providing nucleation sites for the precipitating chalk [6]. Water exposed to atmospheric CO2 pressure can only keep about 47 mg/l calcium carbonate (chalk) in solution[7]. This amounts to an alkalinity of about 50 ppm as CaCO3 which is less than the lowest possible alkalinity level cited above and as a result allowing amospheric CO2 to dissolve in the water while it is standing does not risk re-dissolving the precipitated chalk. If a GH&KH test kit is available the final hardness and alkalinity achieved through boiling can be checked and used for the estimation of the mash pH (see At home water testing). While the aforementioned process is simple, the need to boil the water represents a great deal of wasted energy. The excess CO2 can also be removed from the water through intensive aeration but that process takes a long time. In fact, surface water tends to have low temporary hardness because due to the lower CO2 content in air the water’s CO2 content is much lower than that possible in ground water and as a result this water cannot hold onto as much chalk as ground water. A more practical approach, that is used by many breweries, is water treatment with slaked lime. The slaked lime is able to absorb both the water’s CO<sub<2</sub> and raise the water pH to transform the bicarbonate into carbonate:
How to conduct this water treatment and calculate the amount of lime needed has been described in detail in Alkalinity reduction with slaked lime and will not be discussed further. Both boiling and slaked lime treatment will only remove bicarbonate and calcium (to some extend magnesium as well) but cannot remove sodium, chloride or sulfate. Those minerals do not affect pH but if a lower concentration is desired the only practical method the home brewer has for their removal is dilution with low mineral water or building brewing water from scratch. Building brewing water from scratchUsing low mineral water like distilled or reverse osmosis water and adding back measured amounts of various salts is becoming increasingly popular among home brewers. One of the likely reasons is that reverse osmosis (commonly abbreviated as RO) units have become reasonably affordable. Many grocery stores also sell RO filtered water for as low as 25 cents for the gallon. Another factor is that this process provides ultimate control over the brewing water composition. While being enthusiastically embraced by home brewers it is not as economical for larger brewers since the production of 1 liter of reverse osmosis water may require as much as 6 liters raw water though the efficiency of these units largely depends on the mineral content of the raw water and the design of the unit. Reverse osmosis works by pushing water through a filter membrane that has openings large enough for water but not for mineral ions. The result is lower mineral water, the product water, on one side and higher mineral water, the waste water, on the other side. The process is slow and RO units are generally equipped with storage tanks that deliver water when needed. For residential under counter units these tanks generally hold only 1.5 - 1.7 gallons which is too little to supply the water needed in brewing. An upgrade to a 7 gallon or even larger tank is recommended. The mineral content of the water should regularly be tested with a TDS (Total Dissolves Solids) meter. These meters measure the electric resistance of the water to determine its mineral contend and tend to be included when a RO unit is purchased. An increase in the RO water's mineral content means that it is time to replace the membrane. I have been using my RO unit for about 4 years now and have not had the need to replace the membrane. RO water is not completely free of minerals. About 5 - 10% of the initial mineral content remains but the resulting water is low enough in minerals that it can be treated as being free of minerals. If so desired you may also send a sample of the RO water to a water analysis lab to get a base water profile for your brewing water additions. When building water from scratch brewers should have the following salts on hand:
Baking soda and Epsom salt can be found in the grocery store. The other salts are best purchased at a home brewing store. A digital scale for precisely measuring grams of salts is also very helpful. I have had very good success with jewelry scales (100-200g capacity, 0.01g resolution) that can be found on e-bay for ~$20. Water modification spreadsheetCalculating and predicting the results of water modification can be complicated due to the chemistry involved. To simplify these calculations a water calculation spread sheet has been developed. This spreadsheet supports:
Designing and modifying brewing waterUsing a water spreadsheet and determining the necessary water modifications is one of the more daunting aspects or water chemistry for brewers that want to start taking charge of their water. There are just too many variables that can be changed. Here are some guidelines that can help:
Various water recipes lists a few water recipes that have worked well for me in the past. Historic and current water profiles of major brewing cities may also be used as a guideline for a particular beer. But it is important to understand that these waters may have undergone water treatment before being used to brew a style that originated from that region. If the historic water profile fits the beer that you are brewing, i.e. the mash pH is in an acceptable range, you may want to target this water since brewers may most likely have been using this water for their beers. A classic example for this is the water profile for Munich. While this water is great for brewing a Munich Dunkel or a Doppelbock it is the wrong water for brewing a Munich Helles. This largely Pilsber malt based beer requires water with low residual alkalinity. To get to the desired mash pH Munich brewers likely precipitate alkalinity from the water using lime and in addition to that use sour fermented wort (Sauergut) to correct the mash pH even lower. The Paulaner brewery, for example, doesn't even have to decarbonate their water. They are getting very soft (~ 2 dH, 40 ppm as CaCO3) brewing water from a deep well on their property. | |
Salt and acid additions to the mashMash pH and later wort pH can also be adjusted on the fly. This is done after the pH has been tested and found to be too high or too low by adding either salts or acids to bring the pH back into range. Since the malt is the dominating pH buffer the calculation of the necessary acid or salts are best based on the grist weight while the mash thickness (i.e. the amount of mash water used) matters little. All salts and acids that can be added to the water can also be added to the mash. If salts are added only to the mash their effective concentration in the beer will be lower since the additional water brought in during sparging will dilute the mineral concentrations in the kettle. While it is practical to threat the water and the grist such that they achieve the desired mash pH, many brewers find it easier to measure the pH of a mash sample 5-10 min after dough-in and base the addition of salts and acids based on how far away this pH is from the target pH. This works particularly well for brewers who rarely repeat recipes and thus have less historical data that could be used to estimate the necessary water and mash treatments. While there doesn’t appear to be any difference between treating the water and treating the mash with respect to the pH the mash will settle at, there can be a slight difference in expression of enzymatic activity. In particular in single infusion mashing where the bulk of the fermentable sugars is created by beta-amylase early during the mash before large amounts of that enzyme fall victim to the mash temperature. This enzyme has a pH optimum between 5.3 - 5.6 [8] and if the pH is sufficiently far off this optimum during the first 10-20 min the amount of fermentable sugars produced and with it the fermentablility of the created wort may suffer. |
|
References
|