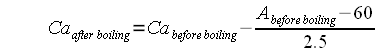Difference between revisions of "Mash pH control"
| (82 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{| style="width:850px" border="0" | {| style="width:850px" border="0" | ||
| − | + | | | |
| − | + | ||
| − | + | ||
<div style="clear:both;"></div> | <div style="clear:both;"></div> | ||
| − | This is the 3rd | + | This is the 3rd article in a series of articles intended to educate the interested brewer about pH in brewing. Part 1, [[An Overview of pH]], showed the basic principles behind pH and how it can be measured, part 2, [[How pH affects brewing]], illustrated the effects pH has on various brewing processes and which pH ranges are considered optimal. This article will be the most practical. It shows how malt and water settle at a pH and how this pH can be affected through changes in water composition, malt bill and mash additions. For the interested brewer, it also goes behind the scenes and explains the chemical processes that are at work. A number of water treatment and brewing water building options are explained. |
| + | |||
=Water, malt and mash pH= | =Water, malt and mash pH= | ||
| + | |- | ||
| + | | | ||
| − | Water and malt are both pH buffers, that means they have their own pH and a desire to resist pH changes. When they are brought together at dough-in, they will settle at a pH which will be the mash pH. Which pH they'll settle at depends on how strong either of the components (water and malt) pull | + | Water and malt are both pH buffers, that means they have their own pH and a desire to resist pH changes. When they are brought together at dough-in, they will settle at a pH which will be the mash pH. Which pH they'll settle at depends on how strong either of the components (water and malt) pull pH to their respective sides. In this "tug of war" malt is the acidic one, it wants to lower pH, and water tends to be the alkaline component, it wants to raise pH. |
| − | As we have seen in [[How_pH_affects_brewing#enzymatic_activity|Enzymatic Activity]] the mash pH range that works for brewing is fairly wide. 5.0 – 6.0 will work with most enzymatically strong malts and 5.3-5.5 is considered optimal. This wide range of possible mash pH values is the reason why most brewers don't have to worry about mash pH and water chemistry at the beginning of their home brewing career. As we will see later, average water and grist compositions generally found in (home) brewing are likely to result in a mash pH in that range. Only at the extremes will brewers experience problems in mash conversion and off flavors that arise from incorrect mash pH levels | + | As we have seen in [[How_pH_affects_brewing#enzymatic_activity|Enzymatic Activity]] the mash pH range that works for brewing is fairly wide. 5.0 – 6.0 will work with most enzymatically strong malts and 5.3-5.5 is considered optimal. This wide range of possible mash pH values is the reason why most brewers don't have to worry about mash pH and water chemistry at the beginning of their home brewing career. As we will see later, average water and grist compositions generally found in (home) brewing are likely to result in a mash pH in that range. Only at the extremes will brewers experience problems in mash conversion and off flavors that arise from incorrect mash pH levels which spark the brewer's interest in that rather technical topic. |
But even if you don't experience off flavors or mash problems from incorrect mash pH, there is likely a point in your brewing career where the beers are good and very enjoyable but you want to take them to excellent. Controlling your mash pH and moving it into a more optimal range has the potential to do that for you. The effects that pH has in brewing were elaborated in [[How pH affects brewing]] and they start with a proper mash pH. | But even if you don't experience off flavors or mash problems from incorrect mash pH, there is likely a point in your brewing career where the beers are good and very enjoyable but you want to take them to excellent. Controlling your mash pH and moving it into a more optimal range has the potential to do that for you. The effects that pH has in brewing were elaborated in [[How pH affects brewing]] and they start with a proper mash pH. | ||
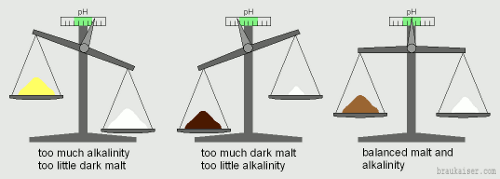
| − | [[Image:Malt_and_alkalinity_scales.gif|frame|right|Figure 1 - | + | [[Image:Malt_and_alkalinity_scales.gif|frame|right|Figure 1 - Balancing malt and water is, in its simplest form, like balancing a scale]] |
| − | A correct mash pH is all about matching the right water with the right grist and possibly the addition of some acid. Since we brewers generally start with a style of beer or recipe in mind, the grist composition is known and we want to know what kind of water modifications are | + | A correct mash pH is all about matching the right water with the right grist and possibly the addition of some acid. Since we brewers generally start with a style of beer or recipe in mind, the grist composition is known and we want to know what kind of water modifications are needed to get the desired mash pH. In a way this is like balancing a scale (Figure 1). On the left is the malt and on the right is the water. But we are not interested in their actual weight. Instead we want to know how acidic is the malt is and how alkaline is the water. |
| − | To determine how acidic malt is, I ran | + | To determine how acidic malt is, I ran experiments were I tested the distilled water mash pH of some base malts and the acidity of various specialty malts. And what I found was that darker malts are generally more acidic than lighter colored malts. While there were a few exceptions to that rule, which we will examine later, we will work with that for now: The darker malts in the grain bill, the more acidic the grist is. |
| − | That acidity of the malt is counteracted by the alkalinity of the water. Water alkalinity measures the waters ability to neutralize acids and thus resist a change in pH. In fact, it is measured by adding a strong acid to a sample of water until it reaches a pH of 4.3 | + | That acidity of the malt is counteracted by the alkalinity of the water. Water alkalinity measures the waters ability to neutralize acids and thus resist a change in pH. In fact, it is measured by adding a strong acid to a sample of water until it reaches a pH of 4.3 <ref name=deLange_alkalinity_I>A.J. deLange, [http://ajdel.wetnewf.org:81/Brewing_articles/BT_alkalinity/alkalinity.pdf Alkalinity Part I]</ref>. The amount of acid needed determines how alkaline the water is. The more acid that is needed, the more alkaline the water is. |
When grist and water are mixed the alkalinity of the water will neutralize some of the malt’s acidity. Neutralization happens when an acid and a base are brought together. The result is a salt and water and a pH that lies between the pH of the acid and the pH of the base. The acidity of the grist is best thought of as its mash pH with distilled water. Alkalinity present in the water or from the addition of alkaline salts pulls that pH higher (more alkaline) while the addition of acid, calcium or magnesium lowers that pH (more acidic). | When grist and water are mixed the alkalinity of the water will neutralize some of the malt’s acidity. Neutralization happens when an acid and a base are brought together. The result is a salt and water and a pH that lies between the pH of the acid and the pH of the base. The acidity of the grist is best thought of as its mash pH with distilled water. Alkalinity present in the water or from the addition of alkaline salts pulls that pH higher (more alkaline) while the addition of acid, calcium or magnesium lowers that pH (more acidic). | ||
If, for example, the grist has low acidity because it contains only lightly colored malts and the water has a lot of alkalinity, the mash pH will be higher than desired. If the opposite is true, lots of malt acidity from dark grains and low alkalinity water, the mash pH will be lower than desired. If malt acidity and water alkalinity are balanced, the pH will be in the desired range. | If, for example, the grist has low acidity because it contains only lightly colored malts and the water has a lot of alkalinity, the mash pH will be higher than desired. If the opposite is true, lots of malt acidity from dark grains and low alkalinity water, the mash pH will be lower than desired. If malt acidity and water alkalinity are balanced, the pH will be in the desired range. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_basics.gif|link=|alt={Brewing Basics}]] | ||
| + | |- | ||
| + | | | ||
== Mash as a pH buffer == | == Mash as a pH buffer == | ||
| − | Grist, water, acid and salt interactions within the mash are best thought of as the addition of acids or bases to a strong buffer which is largely formed by phosphates, proteins and amino acids present in the malt. Dealing with a buffered system means that the amount of acid or base needed to change | + | Grist, water, acid and salt interactions within the mash are best thought of as the addition of acids or bases to a strong buffer which is largely formed by phosphates, proteins and amino acids present in the malt. Dealing with a buffered system means that the amount of acid or base needed to change pH depends largely on the amount of buffer (in this case weight of the grist) and the desired pH change. The amount of acid it takes to lower or raise the mash pH of 1 kg of grist by 0.1 pH units is about 3-5 mEq. [[A simple Model for pH Buffers]] shows how we can visualize pH buffers as differently shaped vertical tubes. |
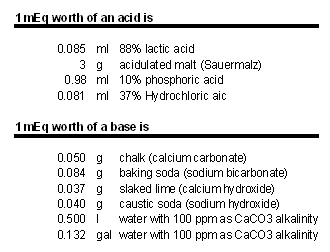
[[Image:MEq_to_acid_base.gif|frame|right|Table 1 - Examples for 1 mEq worth of acid and base. A pH of ~5.5 is assumed]] | [[Image:MEq_to_acid_base.gif|frame|right|Table 1 - Examples for 1 mEq worth of acid and base. A pH of ~5.5 is assumed]] | ||
| − | mEq (milliequivelent) or Eq (1 mEq = 0.001 Eq) is a unit commonly used to express the amount of acid or | + | mEq (milliequivelent) or Eq (1 mEq = 0.001 Eq) is a unit commonly used to express the amount of acid or base. Rather than giving the specific amount of acid or base needed it specifies the number of hydrogen ions that are added (acid) or absorbed (base). To convert Eq to an actual amount of acid/base its molar weight, number of hydrogen ions donated/absorbed and its concentration needs to be known. Lactic acid for example has a molar weight of 90 g/mol. Above a pH of 5 almost all its molecules donate 1 hydrogen ion. This means 90g 100% lactic acid contribute about 1 Eq acid. The lactic acid we commonly use has a concentration of 88% by weight and 1 Eq of that weighs 102 g. 1 mEq weighs 0.102 g. |
Calcium carbonate (chalk), on the other hand, has a molar weight of 100 g/mol but can absorb 2 hydrogen ions. To get 1 Eq worth of acid neutralizing power 50g chalk are needed. In brewing that neutralizing power will be lower due to the acidifying effect of the calcium when it reacts with the malt’s phosphates. But more about this later. | Calcium carbonate (chalk), on the other hand, has a molar weight of 100 g/mol but can absorb 2 hydrogen ions. To get 1 Eq worth of acid neutralizing power 50g chalk are needed. In brewing that neutralizing power will be lower due to the acidifying effect of the calcium when it reacts with the malt’s phosphates. But more about this later. | ||
| Line 40: | Line 45: | ||
Table 1 on the right shows the amounts of a few acids and bases that provide 1 mEq of neutralizing power. | Table 1 on the right shows the amounts of a few acids and bases that provide 1 mEq of neutralizing power. | ||
| − | The buffer capacity of the malt, which affects the amount of acid or base needed to change | + | The buffer capacity of the malt, which affects the amount of acid or base needed to change mash pH, does to some extend depend on the malting process as well as the mashing process. This may lead to differences how the mash responds to pH correction efforts and makes exact mash pH predictions difficult. |
| − | =Factors | + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] |
| + | |- | ||
| + | | | ||
| + | |||
| + | =Factors affecting mash pH= | ||
The following is a more detailed discussion of what affects mash pH in brewing. | The following is a more detailed discussion of what affects mash pH in brewing. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_inner_workings.gif|link=|alt={How Things Work}]] | ||
| + | |- | ||
| + | | | ||
==Grist== | ==Grist== | ||
| − | [[Image: | + | [[Image:Charts_base_malt_pH_and_malt_acidity.gif|frame|center|Figure 2 - '''On the left''': the mash pH of mashes with various base when mashed with distilled water. The 2-row that falls out of line for the pale malts is Rahr 2-row. '''On the right''': The titratable acidity of various specialty malts plotted over their color. The test mashes made from these malts were titrated to a pH of 5.7]] |
| − | + | Very lightly kilned malts have a natural pH between 5.7 and 5.8. Wheat malts may even be higher than that. Once malts are kilned more intensely to produce darker base malts, the distilled water pH drops due to the formation of acidic melanoidins. This is shown in Figure 2 which plots the color and distilled water mash pH for a number of different base malt samples. There is a loose correlation between malt color and mash pH: "The darker the malt the lower the mash pH". Because of this loose correlation the prediction of the malts mash pH based on its color is difficult in particular for darker malts. | |
| − | + | Specialty malts like crystal and roasted malts are kilned to even darker colors than base malts. While they may constitute a much smaller portion of the grist they tend contribute a large portion of the grist’s color and with it acidity. The latter lowers the grist pH below the pH of the base malts used. | |
| − | + | The acidity of various specialty malts is plotted over their color in Figure 2. Crystal type malts adhere fairly strongly to the "the darker the more acidic" rule. Roasted malts, on the other hand, showed a fairly constant acidity regardless of their color. This is assumed to be the result of differences in the production process for crystal and roasted malts. The color of crystal malts is created while the malt is still wet which allows for the formation of more acidic compounds while the color of roasted malts is created after they have already been dried. Though that creates a stronger color it creates less acidity<ref name=troester>Kai Troester, [http://braukaiser.com/documents/effect_of_water_and_grist_on_mash_pH.pdf The effect of brewing water and grist composition on the pH of the mash], 2009</ref> | |
| − | + | For us brewers this means that grists containing large amounts of roasted malts are likely less acidic than grists containing large amounts of dark crystal malts even though the beers made with roasted malts might be darker in color. | |
| − | + | |- | |
| + | | | ||
| − | + | [[Image:Beer_color_to_DI_pH.gif|frame|center|Figure 3 - The predicted distilled water mash pH of 210 randomly generated grists]] | |
| + | |||
| + | To get a better understanding what crystal and roasted malts do to the acidity of the grist I randomly generated 210 different grist compositions using the malts I analyzed and the following constraints: | ||
| + | |||
| + | * 15 recipes mixed only base malts | ||
| + | * 45 recipes mixed base malts with up to 15% crystal malts. 50 of these recipes used only 2-row as base malt. The other 15 used a random mix of different base malts. The percentage of crystal malts in these grists was randomly chosen between 1 and 15%. In addition to that the types and and percentages of different crystal malts were also chosen randomly | ||
| + | * 45 recipes mixed base malts with up to 8% crystal malts and up to 8% roasted malts. 50 of these recipes used only 2 row as base malt while the other 15 used a mix of base malts. Similar to the recipes that used only crystal malts as specialty malts these recipes here had their actual crystal and roasted malt percentages determined randomly. The same applied to the types of malts that were chosen | ||
| + | * 45 recipes used only roasted malts. These recipes were created similar to the crystal only recipes mentioned earlier except that they only used roasted malts as base malts. | ||
| + | |||
| + | The beer color for all these beers was calculated and mash pH was estimated based on the malt data available. The mash pH was then plotted over the beer color and is shown in Figure 3. It is apparent that the majority of the beers falls into the desired mash pH range if the water has no residual alkalinity (What residual alkalinity is will be explained later in this article). If the waater has moderate residual alkalinity the mash pH will be slightly but not dramatically higher. This is the reason why most average beers can be brewed well with average water. Only at the extremes would be mash pH become a problem. | ||
| + | |||
| + | Another observation in this figure is that there is a somewhat linear relationship between beer color and mash pH. The slope of that line is steeper for beers that get all or most of their color from crystal malts and flatter for beers that get their color from roasted malts. This relationship can be used to predict grist acidity from the beer color and the percentage of color that is coming from roasted malt. Eventually this can be used to predict mash pH and such an algorithm is described in [[Beer color, alkalinity and mash pH]] and has been implemented in the [http://braukaiser.com/downloads/Kaiser_water_calculator.xls Kaiser_water_calculator.xls]. | ||
| + | |||
| + | | valign="top" | [[Image:Icon_science.gif|link=|alt={Geeky Stuff}]] | ||
| + | |- | ||
| + | | | ||
==Water minerals== | ==Water minerals== | ||
| − | The minerals listed here may either come from | + | The minerals listed here may either come from the base water, from salts added to the water before dough-in or salts added to the mash. Experiments have shown that the source of the minerals does not make much of a difference in their effect on mash pH. |
| − | What makes a difference though, is the amount of minerals that are brought into the mash. This amount depends on both mash thickness (how much water per unit of grain) and the mineral concentration in the water. In other words, water in a thin mash will be able to have | + | What makes a difference though, is the amount of minerals that are brought into the mash. This amount depends on both mash thickness (how much water per unit of grain) and the mineral concentration in the water. In other words, water in a thin mash will be able to have larger effect on mash pH compared to the same water water in a thick mash since in the latter case there will be less water, and with it less minerals, per grain. |
| − | This being said, brewers should not | + | This being said, brewers should not conclude that thick mashes provide a practical means of dealing with high alkalinity water just because the alkalinity won’t be able to move the mash pH as much. Any water that is not used in the mash is used during the sparge where it is able to adversely affect pH in both the grain bed and boil kettle. |
| + | |||
| + | This section focuses only on the water minerals which have an effect on pH. A more complete discussion of brewing water composition can be found in [[How to read a water report]]. | ||
===Bicarbonate and Alkalinity=== | ===Bicarbonate and Alkalinity=== | ||
| − | + | As mentioned in the beginning, alkalinity is the water’s ability to neutralize acids and the ions involved with that neutralization are bicarbonate (HCO<sub>3</sub><sup>-</sup>) and carbonate (CO<sub>3</sub><sup>2-</sup>). More info about carbonate chemistry can be found in [[Building_brewing_water_with_dissolved_chalk#About_chalk_and_the_carbonate_system|About chalk and the carbonate system]] | |
| − | When brought in contact with the more acidic malt the bicarbonate and carbonate ions absorb hydrogen ions which results in a pH rise. The extent of this pH increase largely depends on the amount of malt, amount of water and the bicarbonate and carbonate concentration in that water. The latter is expressed by the water’s alkalinity. The more bicarbonate and carbonate ions there are per unit of malt the more the pH will rise. When bicarbonate or carbonate | + | When brought in contact with the more acidic malt the bicarbonate and carbonate ions absorb hydrogen ions which results in a pH rise. The extent of this pH increase largely depends on the amount of malt, amount of water and the bicarbonate and carbonate concentration in that water. The latter is expressed by the water’s alkalinity. The more bicarbonate and carbonate ions there are per unit of malt the more the pH will rise. When bicarbonate or carbonate neutralizes an hydrogen ion it forms carbon dioxide. While bicarbonate is able to neutralize one hydrogen ion carbonate can neutralize two: |
| − | H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> → H<sub>2</sub>O + CO<sub>2</sub> | + | :H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> → H<sub>2</sub>O + CO<sub>2</sub> |
| − | 2 H<sup>+</sup> + CO<sub>3</sub><sup>2-</sup> → H<sub>2</sub>O + CO<sub>2</sub> | + | :2 H<sup>+</sup> + CO<sub>3</sub><sup>2-</sup> → H<sub>2</sub>O + CO<sub>2</sub> |
| − | Due to the poor solubility of calcium carbonate and the presence of | + | Due to the poor solubility of calcium carbonate and the presence of calcium in most waters, appreciable amounts of carbonate are rarely found in brewing water. As a result the bicarbonate concentration in water can be calculated from the alkalinity of the water and vice versa. Carbonate ions will be present when chalk is added to the water or the mash. The addition of chalk, however, will be discussed later. |
===Calcium and Magnesium=== | ===Calcium and Magnesium=== | ||
| − | Brewing water also contains calcium and magnesium ions. These ions are able to react with phosphates from the malt to form insoluble phosphate salts which precipitate. At mash pH values between 5 and 6 most of the phosphate is available as HPO<sub>4</sub><sup>2-</sup>. The reaction with calcium liberates hydrogen ions which react acidic and lower the pH of the mash | + | Brewing water also contains calcium and magnesium ions. These ions are able to react with phosphates from the malt to form insoluble phosphate salts which precipitate. At mash pH values between 5 and 6 most of the phosphate is available as HPO<sub>4</sub><sup>2-</sup>. The reaction with calcium liberates hydrogen ions which react acidic and lower the pH of the mash <ref name="Narziss_Back">Ludwig Narziss, Werner Back, Die Bierbrauerei Band 2: Technologie der Würzebereitung, Wiley-VCH, 2009</ref>: |
| − | 3Ca<sup>2+</sup> + 2 HPO<sub>4</sub><sup>2-</sup> → 3 Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> ↓ + 2 H<sup>+</sup> | + | :3Ca<sup>2+</sup> + 2 HPO<sub>4</sub><sup>2-</sup> → 3 Ca<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> ↓ + 2 H<sup>+</sup> |
Magnesium shows a similar but less effective reaction with phosphates which is why it has only half the pH lowering power of calcium. | Magnesium shows a similar but less effective reaction with phosphates which is why it has only half the pH lowering power of calcium. | ||
| − | Malt contains about 1 % of phosphate by weight | + | Malt contains about 1 % of phosphate by weight<ref name ="deLange_phosphate">A.J. deLange, [http://ajdel.wetnewf.org:81/Brewing_articles/Cerevesia/Final_galley Alkalinity, Hardness, Residual Alkalinity and Malt Phosphate: Factors in the Establishment of Mash pH], 2004</ref> . About 80% of that end up in the wort. This amount is far greater than the calcium or magnesium that is brought in with the brewing liquor which makes calcium and magnesium the limiting factor. |
As early as 1914 did the German brewing scientist Windish show that water ions have an effect on the mash pH. Kolbach, another German brewing scientist, later showed that it takes about 1.75 calcium ions and twice as many magnesium ions to produce one hydrogen ion. (1.75 calcium ions equal 3.5 calcium equivalents) | As early as 1914 did the German brewing scientist Windish show that water ions have an effect on the mash pH. Kolbach, another German brewing scientist, later showed that it takes about 1.75 calcium ions and twice as many magnesium ions to produce one hydrogen ion. (1.75 calcium ions equal 3.5 calcium equivalents) | ||
| − | My own research on this subject reported slightly lower numbers: 1.3-1.5 Calcium ions and 2.4-2.8 magnesium ions | + | My own research on this subject reported slightly lower numbers: 1.3-1.5 Calcium ions and 2.4-2.8 magnesium ions<ref name="troester"/>. It should be noted that Kolbachs work was primarily targeted at the cast out wort pH and not so much mash pH. There is however a close correlation between the two. |
===Residual Alkalinity=== | ===Residual Alkalinity=== | ||
| − | The acidic reaction of calcium and magnesium counteracts the alkaline reaction of the water’s alkalinity, which prompted Kolbach to define the residual alkalinity as the alkalinity that remains after the calcium and magnesium reactions have been considered. Based on his work the following formula for residual alkalinity RA has been established in the brewing world: | + | The acidic reaction of calcium and magnesium counteracts the alkaline reaction of the water’s alkalinity, which prompted Kolbach to define the residual alkalinity as the alkalinity that remains after the calcium and magnesium reactions have been considered. Based on his work the following formula for residual alkalinity RA has been established in the brewing world <ref name="Kolbach">Paul Kohlbach, Brewing Chemistry and Technology Department, VLB, Berlin Scientific Appendix, Monthly for Brewing, Paul Kohlbach, Editor, Vol 6 Number 5 May |
| + | 1953 Berlin [http://ajdel.wetnewf.org:81/Brewing_articles/KolbachPaper.pdf translation curtesy of A.J. deLange]</ref> | ||
[[Image:Formula_RA.gif]] | [[Image:Formula_RA.gif]] | ||
Where: | Where: | ||
| − | * RA: residual alkalinity given as an [[How to read a water report|equivalent | + | * RA: residual alkalinity given as an [[How to read a water report|equivalent unit]] (mEq/l, ppm as CaCO<sub>3</sub>, dH) |
| − | * A: alkalinity given in the same equivalent | + | * A: alkalinity given in the same equivalent unit |
| − | * CH: calcium hardness of the water, which is the calcium ion concentration given in an equivalent | + | * CH: calcium hardness of the water, which is the calcium ion concentration given in an equivalent unit |
| − | * MH: magnesium hardness of the water, which is the magnesium ion concentration given in an equivalent | + | * MH: magnesium hardness of the water, which is the magnesium ion concentration given in an equivalent unit |
The residual alkalinity is a property of the brewing water and allows brewers to estimate a water’s effect on the mash pH. | The residual alkalinity is a property of the brewing water and allows brewers to estimate a water’s effect on the mash pH. | ||
| Line 113: | Line 145: | ||
[[Image:Formula_RA_from_GH.gif]] | [[Image:Formula_RA_from_GH.gif]] | ||
| − | This makes the assumption that about 30% of the water hardness comes from magnesium and the remaining 70% come from calcium, which | + | This makes the assumption that about 30% of the water hardness comes from magnesium and the remaining 70% come from calcium, which tends to be the average split between calcium and magnesium hardness in typical waters (see [[At_home_water_testing#Estimating_Residual_Alkalinity|Estimating Residual Alkalinity]]). |
| − | Water with a residual alkalinity of 0 gives about the same mash pH as distilled water while water with a RA greater than 0 yields a higher mash pH. If the water’s alkalinity is low but its calcium and magnesium levels are high the residual alkalinity can | + | Water with a residual alkalinity of 0 gives about the same mash pH as distilled water while water with a RA greater than 0 yields a higher mash pH. If the water’s alkalinity is low but its calcium and magnesium levels are high the residual alkalinity can be less than 0. In this case the use of that water will yield a lower mash pH. |
| + | |||
| + | It should be noted that Kolbach's work focused on the pH of the knock-out wort and not mash pH. Wile there is a close relation his finding that a residual alkalinity change of 10 dH (degrees German hardness, about 171 ppm as CaCO<sub>3</sub>) causes a 0.3 pH change in pH applies to a 12 Plato cast out wort and not mash pH. The change in mash pH will be less and also depends on mash thickness. | ||
| + | |||
| + | [[Residual Alkalinity illustrated]] explains the concept of residual alkalinity through a few drawings of ions in the water rather than through chemical formulas alone. | ||
==Acids== | ==Acids== | ||
| − | We may also add acids to the water or mash to lower | + | We may also add acids to the water or mash to lower mash pH below the pH that is established by water and malt alone. This is in particular necessary for lighter beers where the grist itself is not acidic enough to establish the correct pH. |
| − | Acids donate hydrogen ions which neutralize the alkalinity of the water. | + | Acids donate hydrogen ions which neutralize the alkalinity of the water. Once all the water's alkalinity is consumed the surplus of hydrogen ions counts towards lowering the mash pH. |
==Other factors== | ==Other factors== | ||
| − | The mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates | + | The mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates <ref name="Narziss_Back"/>. |
| + | |||
| + | A rest between 30 and 40 C (90-110 F), known as acid rest, promotes phytase activity which releases more phosphates into the mash. While this is said to acidify the mash I have not observed a significant mash pH decrease though the use of this rest. | ||
| + | |||
| + | |- | ||
| + | | | ||
=Strategies for affecting mash pH= | =Strategies for affecting mash pH= | ||
| Line 135: | Line 176: | ||
* designing water from scratch | * designing water from scratch | ||
* addition of acids or salts to the mash | * addition of acids or salts to the mash | ||
| + | |||
| + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | ||
| + | |- | ||
| + | | | ||
==Changing the grist== | ==Changing the grist== | ||
| − | There was a time when brewers didn't know about water chemistry. They used the local water | + | There was a time when brewers didn't know about water chemistry. They used the available local water and noticed that some beers turned out better than others. This led to the development of local beer styles. But these days we brewers don't want our water to dictate what beer styles we should brew. As a result changing the grist is rarely seen as a viable option. |
The only case where a grist change is practical is the use of acidulated malt or Sauermalz. Acidulated malt is Pilsner malt that has been sprayed with lactic acid before it is dried again. The final lactic acid content of this malt is about 3% by weight. Each 1% of acidulated malt in the grist lowers the mash pH by ~0.1 pH units. More than 4-5% should not be used in order to prevent excessive lactic acid amounts that may be noticeable in the final beer taste. | The only case where a grist change is practical is the use of acidulated malt or Sauermalz. Acidulated malt is Pilsner malt that has been sprayed with lactic acid before it is dried again. The final lactic acid content of this malt is about 3% by weight. Each 1% of acidulated malt in the grist lowers the mash pH by ~0.1 pH units. More than 4-5% should not be used in order to prevent excessive lactic acid amounts that may be noticeable in the final beer taste. | ||
| − | The use of Sauermalz is an elegant way of complying with the German purity law (Reinheitsgebot) since its lactic acid has been produced by lactic acid bacteria that are naturally occurring on the surface of the malt. Because of that the mash pH can be corrected and the beer can still be brewed with just water, malt, hops and yeast. Some German brewers believe that if this loophole would not have been found the German purity law would have needed to be amended since the benefits | + | The use of Sauermalz is an elegant way of complying with the German purity law (Reinheitsgebot) since its lactic acid has been produced by lactic acid bacteria that are naturally occurring on the surface of the malt. Because of that the mash pH can be corrected and the beer can still be brewed with just water, malt, hops and yeast. Some German brewers believe that if this loophole would not have been found the German purity law would have needed to be amended since the benefits to beer quality attained from being able to acidify the mash are substantial. |
More info about the use of lactic acid can be found later in [[#Adding_acids|Adding acids]] | More info about the use of lactic acid can be found later in [[#Adding_acids|Adding acids]] | ||
| Line 148: | Line 193: | ||
==Changing the water== | ==Changing the water== | ||
| − | Before the water can be changed we need to know more about the mineral content of the water. [[How to read a water report]] explains in detail the various minerals | + | Before the water can be changed we need to know more about the mineral content of the water. [[How to read a water report]] explains in detail the various minerals found in drinking water. It also explains what to look for in a water report. |
When the water is changed to correct the mash pH it’s residual alkalinity is changed to match the needs of the grist composition used in the recipe. The desired residual alkalinity range can be estimated from the beer’s color. The darker the beer the more residual alkalinity will be needed to counteract the acidity of the grist. | When the water is changed to correct the mash pH it’s residual alkalinity is changed to match the needs of the grist composition used in the recipe. The desired residual alkalinity range can be estimated from the beer’s color. The darker the beer the more residual alkalinity will be needed to counteract the acidity of the grist. | ||
| − | The residual alkalinity’s effect on pH also depends on mash thickness. The thinner the mash the more pronounced the effect of the water’s alkalinity will be. At a mash thickness of | + | The residual alkalinity’s effect on pH also depends on mash thickness. The thinner the mash the more pronounced the effect of the water’s alkalinity will be. At a mash thickness of 2 l/kg (0.95 qt/lb) it takes a residual alkalinity change of about 130 ppm as CaCO<sub>3</sub> (2.6 mEq/l) to change the mash pH by 0.1 pH units while it takes a residual alkalinity change of just 75 ppm as CaCO<sub>3</sub> (1.5 mEq/l) to achieve the same pH change at a mash thickness of 4 l/kg (1.9 qt/lb)<ref name="troester"/>. |
===Raising residual alkalinity (raises mash pH)=== | ===Raising residual alkalinity (raises mash pH)=== | ||
| − | If the water’s residual alkalinity is too low the addition of alkaline salts like calcium carbonate (chalk, CaCO<sub>3</sub>) or sodium bicarbonate (baking soda, NaHCO<sub>3</sub>) or even strong bases like calcium hydroxide (Ca(OH)<sub>2</sub>) raises its residual alkalinity. If calcium carbonate is used it will not dissolve in the water unless CO<sub>2</sub> is added. Though there are ways to [[Building brewing water with dissolved chalk|dissolve | + | If the water’s residual alkalinity is too low the addition of alkaline salts like calcium carbonate (chalk, CaCO<sub>3</sub>) or sodium bicarbonate (baking soda, NaHCO<sub>3</sub>) or even strong bases like calcium hydroxide (Ca(OH)<sub>2</sub>) raises its residual alkalinity. If calcium carbonate is used it will not dissolve in the water unless CO<sub>2</sub> is added. Though there are ways to [[Building brewing water with dissolved chalk|dissolve calcium carbonate]] many brewers simply add it to the brewing water without dissolving it. In experiments I found that dissolved chalk is not only twice as effective in raising the water’s residual alkalinity, undissolved chalk is also not able to raise the mash pH by more than 0.2 pH units. In other words the addition of more than 500 mg/l undissolved calcium carbonate, which is equivalent to a residual alkalinity of about 200 ppm as CaCO<sub>3</sub>, had little or no effect on mash pH<ref name="troester"/>. As shown in [[Beer color, alkalinity and mash pH]] water with a residual alkalinity of more than 200 ppm as CaCO<sub>3</sub> is rarely needed, even for the darkest beers. A.J. deLange, an expert in everything brewing water, believes that while the mash pH is low enough to dissolve chalk, the actual rate of dissolving chalk at this pH happens slowly which reduces it's effectiveness as a means of raisinbg mash pH<ref name="deLange_chalk">A.J. deLange, personal correspondence</ref>. Despite its shortcomings it still remains the most popular salt addition for raising mash pH. In addition to carbonate chalk also adds calcium to the mash which counteracts some of the pH raising power of the carbonate. |
| − | Baking soda, however, is more soluble than chalk and does not show the somewhat unpredictable mash pH behavior that comes with chalk | + | Baking soda, however, is more soluble than chalk and does not show the somewhat unpredictable mash pH behavior that comes with chalk. The drawbacks of adding baking soda is the increase of the water’s sodium content and the lack of calcium which has a number of positive effects on beer quality. |
Another substance that can be used to increase the alkalinity of the brewing water and thus raise the mash pH is calcium hydroxide (pickling lime, slaked lime, CaOH). It dissolves in water more readily than chalk and doesn't show the limits that undissolved chalk has while it also adds calcium to the mash. The only drawback is that it is a caustic substance and needs to be handled with care. It is best added to the mash after dough-in. | Another substance that can be used to increase the alkalinity of the brewing water and thus raise the mash pH is calcium hydroxide (pickling lime, slaked lime, CaOH). It dissolves in water more readily than chalk and doesn't show the limits that undissolved chalk has while it also adds calcium to the mash. The only drawback is that it is a caustic substance and needs to be handled with care. It is best added to the mash after dough-in. | ||
| Line 164: | Line 209: | ||
===Lowering residual alkalinity (lowers mash pH)=== | ===Lowering residual alkalinity (lowers mash pH)=== | ||
| − | In most cases the residual alkalinity of the water is too high for the desired beer color which causes the mash pH to be too high as well. In | + | In most cases the residual alkalinity of the water is too high for the desired beer color which causes the mash pH to be too high as well. In these cases the water’s residual alkalinity needs to be reduced. For that we brewers have a number of options: |
* dilution | * dilution | ||
| Line 180: | Line 225: | ||
* RA<sub>diluted water</sub>: the resulting residual alkalinity | * RA<sub>diluted water</sub>: the resulting residual alkalinity | ||
* RA<sub>base water</sub>: the starting residual alkalinity | * RA<sub>base water</sub>: the starting residual alkalinity | ||
| − | * r : the percentage of | + | * r : the percentage of base water used. |
| − | Since dilution with low mineral water lowers the concentration of all minerals it may be necessary to supplement the resulting water with calcium ions to get their concentration back into the desired range of 50- | + | Since dilution with low mineral water lowers the concentration of all minerals it may be necessary to supplement the resulting water with calcium ions to get their concentration back into the desired range of 50-150 ppm. This should be done with calcium chloride or calcium sulfate (gypsum) in order to avoid adding bicarbonate which would negate the alkalinity lowering effect of dilution. The added benefit of supplementing calcium is an additional residual alkalinity reduction through the acidic reaction between calcium and malt phosphates. |
| − | ===Adding Calcium=== | + | ====Adding Calcium==== |
| − | As already discussed in [[#Calcium and Magnesium]] calcium and magnesium salts are able to lower | + | As already discussed in [[#Calcium and Magnesium]|Calcium and Magnesium] calcium and magnesium salts are able to lower mash pH trough a reaction with phosphates brought in by the malt. This is of particular interest for beer styles that benefit from water with high permanent hardness. In permanently hard water the anions (negatively charged ions) balancing the calcium and magnesium cations are chloride and sulfate which have no effect on the mash pH. This is not true for temporary hard waters where the balancing anion is bicarbonate. |
| − | Examples for these styles of beer are English Ales and Dortmunder Export. A mash water calcium content of 150 mg/l and little or no alkalinity can yield a mash pH drop of 0.1 – 0.2 pH units depending | + | Examples for these styles of beer are English Ales and Dortmunder Export. A mash water calcium content of 150 mg/l and little or no alkalinity can yield a mash pH drop of 0.1 – 0.2 pH units depending on mash thickness. |
| − | Due to its lower effectiveness with respect to changing the mash pH and its lower desired concentration in brewing water, magnesium salts are generally not used to affect mash pH in any meaningful way. | + | Due to its lower effectiveness with respect to changing the mash pH and its lower desired concentration in brewing water, magnesium salts are generally not used to affect mash pH in any meaningful way. |
====Adding acids==== | ====Adding acids==== | ||
| Line 196: | Line 241: | ||
The water’s bicarbonate content, and with it the alkalinity and residual alkalinity, can also be lowered or completely removed through the addition of organic or inorganic acids. The hydrogen ions released from these acids react with the bicarbonate to form carbon dioxide and water: | The water’s bicarbonate content, and with it the alkalinity and residual alkalinity, can also be lowered or completely removed through the addition of organic or inorganic acids. The hydrogen ions released from these acids react with the bicarbonate to form carbon dioxide and water: | ||
| − | H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> → H<sub>2</sub>O + ↑CO<sub>2</sub> | + | :H<sup>+</sup> + HCO<sub>3</sub><sup>-</sup> → H<sub>2</sub>O + ↑CO<sub>2</sub> |
| − | When acids are used to reduce the alkalinity bicarbonate is replaced with the anion provided by the acid. As a result, excessive use of acids, which may seem necessary with very alkaline waters, can lead to an excess of these ions and an adverse effect on taste. To prevent this water alkalinity may first need to be reduced with means that lower the water’s mineral contents like dilution | + | When acids are used to reduce the alkalinity bicarbonate is replaced with the anion provided by the acid. As a result, excessive use of acids, which may seem necessary with very alkaline waters, can lead to an excess of these ions and an adverse effect on taste. To prevent this water alkalinity may first need to be reduced with means that lower the water’s mineral contents like dilution or alkalinity precipitation, for example. |
While many acids can be used to accomplish this task only a few have found practical use for water treatment in brewing. Those acids are: | While many acids can be used to accomplish this task only a few have found practical use for water treatment in brewing. Those acids are: | ||
| − | * '''Lactic acid''' is an organic acid produced by lactic acid bacteria. To brewers it is available as a 88% by weight solution or acidulated malt. Acidulated malt is pilsner malt that has been sprayed with lactic acid and dried. It contains about 3% lactic acid by weight. Lactic acid may also come from a sour mash or sour fermentation. The latter or acidulated malt is the only acid that can be used for mash and wort pH adjustment in Germany. Narziss reports that the use of lactic acid yields in a smoother beer taste compared to the use of organic acids like hydrochloric acid | + | * '''Lactic acid''' is an organic acid produced by lactic acid bacteria. To brewers it is available as a 88% by weight solution or acidulated malt. Acidulated malt is pilsner malt that has been sprayed with lactic acid and dried. It contains about 3% lactic acid by weight. Lactic acid may also come from a sour mash or sour fermentation. The latter or acidulated malt is the only acid that can be used for mash and wort pH adjustment in Germany. Narziss reports that the use of lactic acid yields in a smoother beer taste compared to the use of organic acids like hydrochloric acid <ref name="Narziss"/>. The anions left behind by lactic acid are lactates which can give the beer a sour twang if used in excess. In my own beers I have used as much as 0.25 g lactic acid per liter of beer which amounts to about 4% acidulated malt in the grist without adverse taste effects. |
* '''Hydrochloric acid (a.k.a muriatic acid)''' is a strong inorganic acid which replaces the bicarbonate with chloride and, when used in excess, can give the beer a salty taste. The muriatic acid found in hardware and pool supply stores is not necessarily food grade and should be avoided. If food grade hydrochloric acid is available it can be used for water treatment but great care should be taken when handling it. Unlike lactic acid, hydrochloric acid is a strongly caustic acid that readily reacts with almost anything it comes in contact with, including your skin and eyes. | * '''Hydrochloric acid (a.k.a muriatic acid)''' is a strong inorganic acid which replaces the bicarbonate with chloride and, when used in excess, can give the beer a salty taste. The muriatic acid found in hardware and pool supply stores is not necessarily food grade and should be avoided. If food grade hydrochloric acid is available it can be used for water treatment but great care should be taken when handling it. Unlike lactic acid, hydrochloric acid is a strongly caustic acid that readily reacts with almost anything it comes in contact with, including your skin and eyes. | ||
* '''Sulfuric acid''': This strong inorganic acid replaces bicarbonate with sulfate and would therefore be a good choice for hoppier beer styles. It is however even more aggressive and dangerous to handle than hydrochloric acid which is why it is rarely used by home brewers. | * '''Sulfuric acid''': This strong inorganic acid replaces bicarbonate with sulfate and would therefore be a good choice for hoppier beer styles. It is however even more aggressive and dangerous to handle than hydrochloric acid which is why it is rarely used by home brewers. | ||
* "Phosphoric acid" is a weak inorganic acid that is much saver to handle that hydrochloric or sulfuric acid and is widely used in soft drinks. It replaces bicarbonate with phosphate and therefore increases the phosphate content of the mash. The amount of phosphate added, however, is small compared to the phosphate added by the malt and therefore the use of phosphoric acid should not lead to the precipitation of additional calcium and magnesium. | * "Phosphoric acid" is a weak inorganic acid that is much saver to handle that hydrochloric or sulfuric acid and is widely used in soft drinks. It replaces bicarbonate with phosphate and therefore increases the phosphate content of the mash. The amount of phosphate added, however, is small compared to the phosphate added by the malt and therefore the use of phosphoric acid should not lead to the precipitation of additional calcium and magnesium. | ||
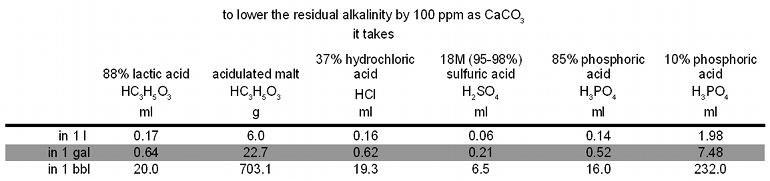
| − | [Image:Acid_and_alkalinity.gif|frame| | + | [[Image:Acid_and_alkalinity.gif|frame|center|Table 2 – The amount of acid it takes to lower the water’s alkalinity, and with it the residual alkalinity, by 100 ppm as CaCO<sub>3</sub>]] |
| − | In order to get the mash pH within the desired 5.3-5.6 range when brewing very pale beers, Pilsners for example, it is generally necessary to use an acid regardless how low the residual alkalinity of the brewing water is. This is because even calcium contents on the high end (150 ppm) and an alkalinity of 0 yields a residual alkalinity of only -70 ppm as CaCO<sub>3</sub>. This residual alkalinity can only lower the mash pH by ~0.2 units when a 4 l/kg (2 qt/lb) mash thickness is used and even less for thicker mashes. With a distilled water mash pH for pilsner malt that tends to be between 5.7 and 5.8 the resulting mash pH would be in the 5.5 to 5.6 range. | + | If more acid than what is needed to neutralize all alkalinity is added, the alkalinity of the water becomes negative. The acid that was not used for neutralizing alkalinity will contribute to lowering the mash pH once the malt is added to the water. |
| + | |||
| + | In order to get the mash pH within the desired 5.3-5.6 range when brewing very pale beers, Pilsners for example, it is generally necessary to use an acid regardless how low the residual alkalinity of the brewing water is. This is because even calcium contents on the high end (150 ppm) and an alkalinity of 0 yields a residual alkalinity of only -70 ppm as CaCO<sub>3</sub>. This residual alkalinity can only lower the mash pH by ~0.2 units when a 4 l/kg (2 qt/lb) mash thickness is used and even less for thicker mashes. With a distilled water mash pH for pilsner malt that tends to be between 5.7 and 5.8 the resulting mash pH without acid additions would only be in the 5.5 to 5.6 range. 2% Sauermalz will get it into the for lighter beers more preferable 5.3-5.4 range. | ||
====Precipitation of calcium carbonate==== | ====Precipitation of calcium carbonate==== | ||
| − | Since the addition of acid does not change the total amount of minerals it may not be suited for the treatment of waters that contain | + | Since the addition of acid does not change the total amount of minerals it may not be suited for the treatment of waters that contain high concentrations of minerals. One method suited for these waters is the precipitation of calcium carbonate through either boiling or the addition of slaked lime. The calcium, and to some extent magnesium, and alkalinity removed through these methods are called temporary hardness. Temporary hardness is the calcium and magnesium ions that can be matched up with bicarbonate ions from the water. In fact, temporary hardness is called temporary because it can be removed through boiling. The remaining calcium and magnesium, which has only sulfate and chloride to pair up with is called permanent hardness. |
| + | |||
| + | When water is boiled CO<sub>2</sub> escapes. This raises the water pH and leads to the creation of more carbonate from the existing bicarbonate. In the presence of calcium the carbonate forms calcium carbonate (a.k.a chalk, CaCO<sub>3</sub>) which is poorly soluble and creates a white precipitate which settles out. If you have water with a high temporary hardness (lots of bicarbonate calcium and magnesium) you may have already noticed this when you cook with your water. | ||
| + | |||
| + | :Ca<sup>2+</sup> + 2HCO<sub>3</sub><sup>-</sup> → ↓CaCO<sub>3</sub> + H<sub>2</sub>O + ↑CO<sub>2</sub> | ||
| + | |||
| + | This method is able to lower the alkalinity to about 50 - 65 ppm as CaCO<sub>3</sub> <ref name=Brungard>Martin Brungard, P.E. D.WRE, [http://www.homebrewersassociation.org/forum/index.php?topic=5792.0 Decarbonation by Boiling]</ref>. The amount of alkalinity that can be removed is the difference between the current alkalinity and the lowest limit (50 - 65 ppm as CaCO<sub>3</sub>) or the calcium hardness, whichever is lower. Sufficient calcium content (i.e. calcium hardness) is needed since the precipitation of 1 ppm alkalinity as CaCO<sub>3</sub> also precipitates 1 ppm calcium hardness as CaCO<sub>3</sub> or 0.4 mg/l calcium. As a result the water may become calcium deficient. | ||
| + | |||
| + | The final calcium level can be calculated with this formula: | ||
| + | |||
| + | [[Image:Formula_Ca_after_boiling.gif]] | ||
| − | + | Where | |
| + | * Ca<sub>after boiling</sub> is the water calcium concentration in mg/l (or ppm) after boiling | ||
| + | * Ca<sub>before boiling</sub> is the initial water calcium concentration in mg/l (or ppm) | ||
| + | * A is the initial water alkalinity in ppm as CaCO<sub>3</sub> | ||
| − | + | If the resulting Calcium concentration is below the recommended minimum of 50 mg/l gypsum or calcium chloride should be added to boost the initial calcium content before the water is boiled. In addition to that the addition of a small amount of chalk facilitates this precipitation by providing nucleation sites for the precipitating chalk <ref name=deLange_Alkalinity>A.J. deLange, [[http://ajdel.wetnewf.org:81/Brewing_articles/BT_Alkalinity_II/AlkalinityPtII.pdf Alkalinity Part II]]</ref>. | |
| − | < | + | Water exposed to atmospheric CO<sub>2</sub> pressure can only keep about 47 mg/l calcium carbonate (chalk) in solution<ref name=calcium_carbonate>Wikipedia, [[http://en.wikipedia.org/wiki/Calcium_carbonate Calcium Carbonate]]</ref>. This amounts to an alkalinity of about 50 ppm as CaCO<sub>3</sub> which is less than the lowest possible alkalinity level cited above and as a result allowing the water to stand for an extended time after it has been boiled does not risk re-dissolving the precipitated chalk though its uptake of atmospheric CO<sub>2</sub>. |
| − | + | If a GH&KH test kit is available the final hardness and alkalinity achieved through boiling can be checked and used for the estimation of the mash pH (see [[At home water testing]]). | |
| − | While the aforementioned process is simple, the need to boil | + | While the aforementioned process is simple, the need to boil a large amount of water represents a great deal of wasted energy. The excess CO<sub>2</sub> can also be removed from the water through intensive aeration but that process takes a long time. In fact, surface water tends to have low temporary hardness because due to the lower CO<sub>2</sub> content in air the water’s CO<sub>2</sub> content is much lower than that possible in ground water and as a result this water cannot hold as much calcium carbonate as ground water. |
| − | A more practical approach that is used by many breweries is water treatment with slaked lime. The slaked lime is able to absorb | + | A more practical approach, that is used by many breweries, is water treatment with slaked lime. The slaked lime is able to absorb the water’s CO<sub<2</sub> and raise the water pH to transform bicarbonate into carbonate: |
| − | Ca<sup>2+</sup> + Ca(OH)<sub>2</sub> + 2HCO<sub>3</sub><sup>-</sup> | + | :Ca<sup>2+</sup> + Ca(OH)<sub>2</sub> + 2HCO<sub>3</sub><sup>-</sup> → ↓2CaCO<sub>3</sub> + H<sub>2</sub>O |
| − | How to conduct this water treatment and calculate the amount of lime needed has been described in detail in [[Alkalinity | + | How to conduct this water treatment and calculate the amount of lime needed has been described in detail in [[Alkalinity reduction with slaked lime]] and will not be discussed further. |
Both boiling and slaked lime treatment will only remove bicarbonate and calcium (to some extend magnesium as well) but cannot remove sodium, chloride or sulfate. Those minerals do not affect pH but if a lower concentration is desired the only practical method the home brewer has for their removal is dilution with low mineral water or building brewing water from scratch. | Both boiling and slaked lime treatment will only remove bicarbonate and calcium (to some extend magnesium as well) but cannot remove sodium, chloride or sulfate. Those minerals do not affect pH but if a lower concentration is desired the only practical method the home brewer has for their removal is dilution with low mineral water or building brewing water from scratch. | ||
| Line 235: | Line 295: | ||
==Building brewing water from scratch== | ==Building brewing water from scratch== | ||
| − | Using low mineral water like distilled or reverse osmosis water and adding back measured amounts of various salts is becoming increasingly popular among home brewers. One of the likely reasons is that reverse osmosis units have become reasonably affordable. Another is that this process provides ultimate control over the brewing water composition. | + | Using low mineral water like distilled or reverse osmosis water and adding back measured amounts of various salts is becoming increasingly popular among home brewers. One of the likely reasons is that reverse osmosis (commonly abbreviated as RO) units have become reasonably affordable. Many grocery stores also sell RO filtered water for as low as 25 cents for the gallon. Another factor is that this process provides ultimate control over the brewing water composition. While being enthusiastically embraced by home brewers it is not as economical for larger brewers since the production of 1 liter of reverse osmosis water may require as much as 6 liters raw water though the efficiency of these units largely depends on the mineral content of the raw water and the design of the unit. |
| + | |||
| + | Reverse osmosis works by pushing water through a filter membrane that has openings large enough for water but not for mineral ions. The result is lower mineral water, the product water, on one side and higher mineral water, the waste water, on the other side. The process is slow and RO units are generally equipped with storage tanks that deliver water when needed. For residential "under counter" units these tanks generally hold only 1.5 - 1.7 gallons which is too little to supply the water needed in brewing. An upgrade to a 7 gallon or even larger tank is recommended. The mineral content of the water should regularly be tested with a TDS (Total Dissolves Solids) meter. These meters measure the electric resistance of the water to determine its mineral contend and tend to be included when a RO unit is purchased. An increase in the RO water's mineral content means that it is time to replace the membrane. I have been using my RO unit for about 4 years now and have not had the need to replace the membrane. | ||
| + | |||
| + | RO water is not completely free of minerals. About 5 - 10% of the initial mineral content remains but the resulting water is low enough in minerals that it can be treated as being free of minerals. If so desired you may also send a sample of the RO water to a water analysis lab to get a base water profile for your brewing water additions. | ||
When building water from scratch brewers should have the following salts on hand: | When building water from scratch brewers should have the following salts on hand: | ||
* gypsum (calcium sulfate) | * gypsum (calcium sulfate) | ||
| − | |||
* calcium chloride | * calcium chloride | ||
* chalk (calcium carbonate) | * chalk (calcium carbonate) | ||
* baking soda (sodium bicarbonate) | * baking soda (sodium bicarbonate) | ||
| + | * Epsom salts (magnesium sulfate) | ||
| − | + | Baking soda can be found in the baking isle and Epsom salt can be found in the health section of grocery stores. The other salts are best purchased at a home brewing store. | |
| − | + | A digital scale for precisely measuring grams of salts is also very helpful. I have had very good success with jewelry scales (100-200g capacity, 0.01g resolution) that can be found on e-bay for ~$20. | |
| − | ==Water modification spreadsheet== | + | ===Water modification spreadsheet=== |
Calculating and predicting the results of water modification can be complicated due to the chemistry involved. To simplify these calculations a [http://braukaiser.com/documents/Kaiser_water_calculator.xls water calculation spread sheet] has been developed. This spreadsheet supports: | Calculating and predicting the results of water modification can be complicated due to the chemistry involved. To simplify these calculations a [http://braukaiser.com/documents/Kaiser_water_calculator.xls water calculation spread sheet] has been developed. This spreadsheet supports: | ||
| − | * the use if simple [[At home water testing|GH&KH]] water analysis results | + | * the use if simple [[At home water testing|GH&KH]] or detailed water analysis results |
* mixing and dilution of water | * mixing and dilution of water | ||
* addition of salts for water treatment including dissolved chalk | * addition of salts for water treatment including dissolved chalk | ||
| − | * addition of slaked lime | + | * addition of slaked lime to raise mash pH |
* addition of lactic acid, acidulated malt and phosphoric acid | * addition of lactic acid, acidulated malt and phosphoric acid | ||
* slaked lime treatment | * slaked lime treatment | ||
* beer color based mash pH prediction | * beer color based mash pH prediction | ||
| − | == Designing brewing water | + | == Designing and modifying brewing water== |
| + | Using a water spreadsheet and determining the necessary water modifications is one of the more daunting aspects or water chemistry for brewers that want to start taking charge of their water. There are just too many variables that can be changed. Here are some guidelines that can help: | ||
| + | * Correct the alkalinity first. This may also require the addition of acids. | ||
| + | * if alkalinity needs to be raised and the sodium content of the base water is low baking soda might be used as long as the sodium level stays below 30-40 mg/l (just something I have done in the past) after that chalk is the better option. | ||
| + | * then make sure that the calcium level is in the desired range. Delicate beers tend to benefit from calcium levels at the low end (50 - 80 mg/l) while more flavorful beers might be better if the water has a calcium content at the upper end of the range (100 - 150 mg/l) | ||
| + | * after that pay attention to chloride and sulfate. Balance hop dominated beers toward sulfate and malt dominated beers toward chloride. In German beers I avoid sulfate levels higher than 90 mg/l even for the more hop dominated Pilsners. | ||
| + | Keep in mind that a salt always add both an anions (-) and a cations (+). You cannot add calcium without also adding chloride, sulfate for alkalinity, for example. This means some tweaking is necessary to get to the desired levels. | ||
| + | |||
| + | [[Various water recipes]] lists a few water recipes that have worked well for me in the past. | ||
| + | |||
| + | Historic and current water profiles of major brewing cities may also be used as a guideline for a particular beer. But it is important to understand that these waters may have undergone water treatment before being used to brew a style that originated from that region. If the historic water profile fits the beer that you are brewing, i.e. the mash pH is in an acceptable range, you may want to target this water since brewers may most likely have been using this water for their beers. | ||
| + | |||
| + | A classic example for this is the water profile for Munich. While this water is great for brewing a Munich Dunkel or a Doppelbock it is the wrong water for brewing a Munich Helles. This largely Pilsber malt based beer requires water with low residual alkalinity. To get to the desired mash pH Munich brewers likely precipitate alkalinity from the water using lime and in addition to that use sour fermented wort (''Sauergut'') to correct the mash pH even lower. The Paulaner brewery, for example, doesn't even have to decarbonate their water. They are getting very soft (~ 2 dH, 40 ppm as CaCO<sub>3</sub>) brewing water from a deep well on their property. | ||
| + | |||
| + | |- | ||
| + | | | ||
==Salt and acid additions to the mash== | ==Salt and acid additions to the mash== | ||
| − | + | Mash pH and later wort pH can also be adjusted on the fly. This is generally done by adding either salts or acids to bring the pH back into the desired range after it has been tested and was found to be too high or too low. | |
| + | |||
| + | Since the malt is the dominating pH buffer the calculation of the necessary acid or salts are best based on the grist weight while mash thickness (i.e. the amount of mash water used) matters little. | ||
| + | |||
| + | All salts and acids that can be added to the water can also be added to the mash. If salts are added only to the mash their effective concentration in the beer will be lower since the additional water used for sparging will dilute the added minerals in the kettle. | ||
| + | |||
| + | While it is practical to treat water and grist such that they achieve the desired mash pH at dough-in, many brewers find it easier to measure the pH of a mash sample 5-10 min after dough-in and base the addition of salts and acids based on how far away this pH is from the targeted pH. This works particularly well for brewers who rarely repeat recipes and thus have less past data that could be used to estimate the necessary water and mash treatments. | ||
| − | + | While there doesn’t appear to be any difference between treating the water and treating the mash with respect to the pH the mash will settle at, there can be a slight difference in expression of enzymatic activity. In particular in single infusion mashing where the bulk of the fermentable sugars is created by beta-amylase early during the mash before large amounts of that enzyme fall victim to the mash temperature. This enzyme has a pH optimum between 5.3 - 5.6 <ref name=Narziss>Prof. Dr. agr. Ludwig Narziss, Prof. Dr.-Ing. habil. Werner Back, ''Abriss der Bierbrauerei'', Technische Universitaet Muenchen (Fakultaet fuer Brauwesen, Weihenstephan). WILEY-VCH Verlags GmbH Weinheim Germany, 2005</ref> and if the pH of the mash is sufficiently far off this optimum during the first 10-20 min the amount of fermentable sugars produced and with it the fermentablility of the created wort may suffer. | |
| − | + | Table 3 contains a summary of the possible pH treatments that can be made to the mash or to the strike water. | |
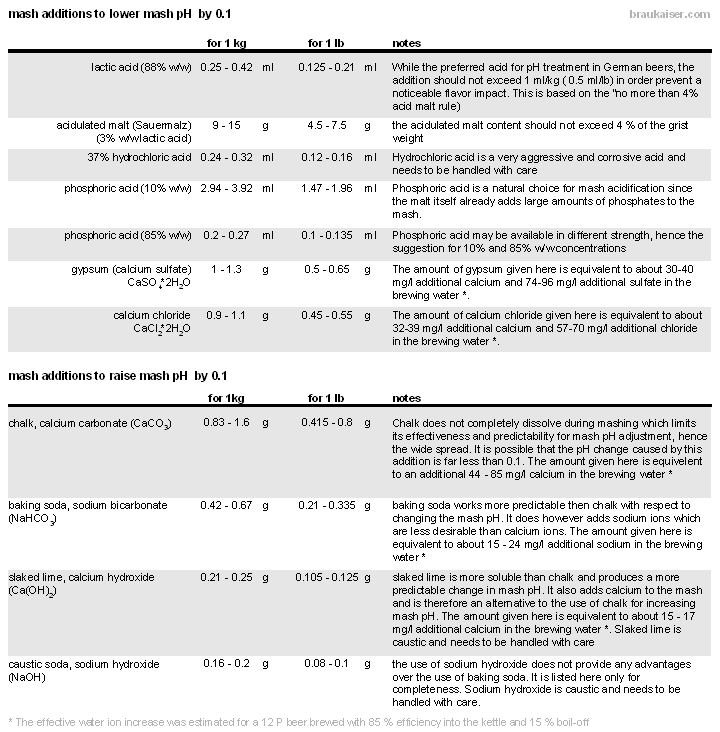
| − | + | [[File:Lowering_raising_mash_pH.gif|frame|center|Table 3 - Guidelines for adding acids and salt to the mash to lower or raise the mash pH by 0.1 pH units. These guidelines have been developed from small scale mash experiments and observations during brewing]] | |
| − | + | | valign="top" | [[Image:Icon_brewing_advice.gif|link=|alt={Practical Brewing Advice}]] | |
| + | |- | ||
| + | | | ||
| + | =References= | ||
| + | <references/> | ||
|} | |} | ||
Latest revision as of 05:14, 4 March 2011
|
This is the 3rd article in a series of articles intended to educate the interested brewer about pH in brewing. Part 1, An Overview of pH, showed the basic principles behind pH and how it can be measured, part 2, How pH affects brewing, illustrated the effects pH has on various brewing processes and which pH ranges are considered optimal. This article will be the most practical. It shows how malt and water settle at a pH and how this pH can be affected through changes in water composition, malt bill and mash additions. For the interested brewer, it also goes behind the scenes and explains the chemical processes that are at work. A number of water treatment and brewing water building options are explained.
ContentsWater, malt and mash pH | |
|
Water and malt are both pH buffers, that means they have their own pH and a desire to resist pH changes. When they are brought together at dough-in, they will settle at a pH which will be the mash pH. Which pH they'll settle at depends on how strong either of the components (water and malt) pull pH to their respective sides. In this "tug of war" malt is the acidic one, it wants to lower pH, and water tends to be the alkaline component, it wants to raise pH. As we have seen in Enzymatic Activity the mash pH range that works for brewing is fairly wide. 5.0 – 6.0 will work with most enzymatically strong malts and 5.3-5.5 is considered optimal. This wide range of possible mash pH values is the reason why most brewers don't have to worry about mash pH and water chemistry at the beginning of their home brewing career. As we will see later, average water and grist compositions generally found in (home) brewing are likely to result in a mash pH in that range. Only at the extremes will brewers experience problems in mash conversion and off flavors that arise from incorrect mash pH levels which spark the brewer's interest in that rather technical topic. But even if you don't experience off flavors or mash problems from incorrect mash pH, there is likely a point in your brewing career where the beers are good and very enjoyable but you want to take them to excellent. Controlling your mash pH and moving it into a more optimal range has the potential to do that for you. The effects that pH has in brewing were elaborated in How pH affects brewing and they start with a proper mash pH. A correct mash pH is all about matching the right water with the right grist and possibly the addition of some acid. Since we brewers generally start with a style of beer or recipe in mind, the grist composition is known and we want to know what kind of water modifications are needed to get the desired mash pH. In a way this is like balancing a scale (Figure 1). On the left is the malt and on the right is the water. But we are not interested in their actual weight. Instead we want to know how acidic is the malt is and how alkaline is the water. To determine how acidic malt is, I ran experiments were I tested the distilled water mash pH of some base malts and the acidity of various specialty malts. And what I found was that darker malts are generally more acidic than lighter colored malts. While there were a few exceptions to that rule, which we will examine later, we will work with that for now: The darker malts in the grain bill, the more acidic the grist is. That acidity of the malt is counteracted by the alkalinity of the water. Water alkalinity measures the waters ability to neutralize acids and thus resist a change in pH. In fact, it is measured by adding a strong acid to a sample of water until it reaches a pH of 4.3 [1]. The amount of acid needed determines how alkaline the water is. The more acid that is needed, the more alkaline the water is. When grist and water are mixed the alkalinity of the water will neutralize some of the malt’s acidity. Neutralization happens when an acid and a base are brought together. The result is a salt and water and a pH that lies between the pH of the acid and the pH of the base. The acidity of the grist is best thought of as its mash pH with distilled water. Alkalinity present in the water or from the addition of alkaline salts pulls that pH higher (more alkaline) while the addition of acid, calcium or magnesium lowers that pH (more acidic). If, for example, the grist has low acidity because it contains only lightly colored malts and the water has a lot of alkalinity, the mash pH will be higher than desired. If the opposite is true, lots of malt acidity from dark grains and low alkalinity water, the mash pH will be lower than desired. If malt acidity and water alkalinity are balanced, the pH will be in the desired range. |
|
Mash as a pH bufferGrist, water, acid and salt interactions within the mash are best thought of as the addition of acids or bases to a strong buffer which is largely formed by phosphates, proteins and amino acids present in the malt. Dealing with a buffered system means that the amount of acid or base needed to change pH depends largely on the amount of buffer (in this case weight of the grist) and the desired pH change. The amount of acid it takes to lower or raise the mash pH of 1 kg of grist by 0.1 pH units is about 3-5 mEq. A simple Model for pH Buffers shows how we can visualize pH buffers as differently shaped vertical tubes. mEq (milliequivelent) or Eq (1 mEq = 0.001 Eq) is a unit commonly used to express the amount of acid or base. Rather than giving the specific amount of acid or base needed it specifies the number of hydrogen ions that are added (acid) or absorbed (base). To convert Eq to an actual amount of acid/base its molar weight, number of hydrogen ions donated/absorbed and its concentration needs to be known. Lactic acid for example has a molar weight of 90 g/mol. Above a pH of 5 almost all its molecules donate 1 hydrogen ion. This means 90g 100% lactic acid contribute about 1 Eq acid. The lactic acid we commonly use has a concentration of 88% by weight and 1 Eq of that weighs 102 g. 1 mEq weighs 0.102 g. Calcium carbonate (chalk), on the other hand, has a molar weight of 100 g/mol but can absorb 2 hydrogen ions. To get 1 Eq worth of acid neutralizing power 50g chalk are needed. In brewing that neutralizing power will be lower due to the acidifying effect of the calcium when it reacts with the malt’s phosphates. But more about this later. Table 1 on the right shows the amounts of a few acids and bases that provide 1 mEq of neutralizing power. The buffer capacity of the malt, which affects the amount of acid or base needed to change mash pH, does to some extend depend on the malting process as well as the mashing process. This may lead to differences how the mash responds to pH correction efforts and makes exact mash pH predictions difficult. |
|
Factors affecting mash pHThe following is a more detailed discussion of what affects mash pH in brewing. |
|
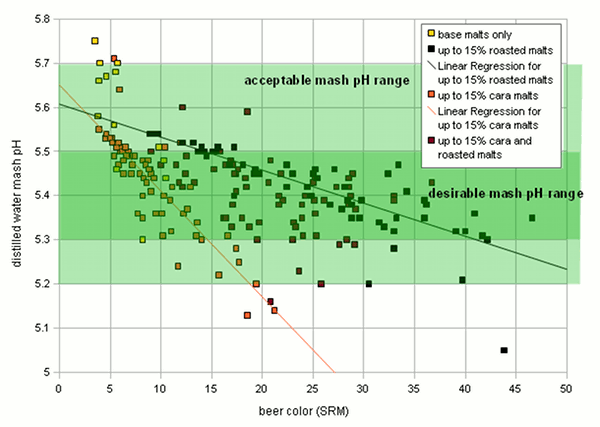
Grist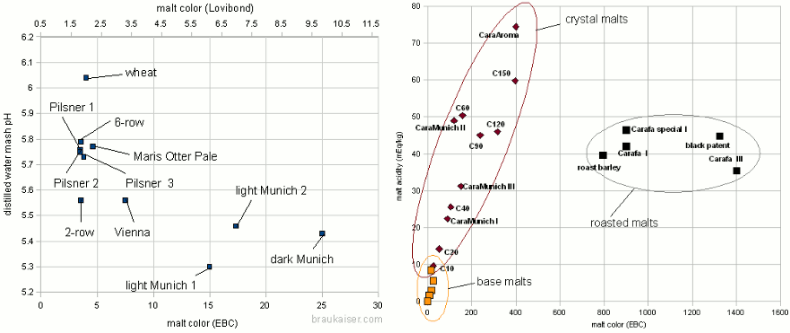 Figure 2 - On the left: the mash pH of mashes with various base when mashed with distilled water. The 2-row that falls out of line for the pale malts is Rahr 2-row. On the right: The titratable acidity of various specialty malts plotted over their color. The test mashes made from these malts were titrated to a pH of 5.7 Very lightly kilned malts have a natural pH between 5.7 and 5.8. Wheat malts may even be higher than that. Once malts are kilned more intensely to produce darker base malts, the distilled water pH drops due to the formation of acidic melanoidins. This is shown in Figure 2 which plots the color and distilled water mash pH for a number of different base malt samples. There is a loose correlation between malt color and mash pH: "The darker the malt the lower the mash pH". Because of this loose correlation the prediction of the malts mash pH based on its color is difficult in particular for darker malts. Specialty malts like crystal and roasted malts are kilned to even darker colors than base malts. While they may constitute a much smaller portion of the grist they tend contribute a large portion of the grist’s color and with it acidity. The latter lowers the grist pH below the pH of the base malts used. The acidity of various specialty malts is plotted over their color in Figure 2. Crystal type malts adhere fairly strongly to the "the darker the more acidic" rule. Roasted malts, on the other hand, showed a fairly constant acidity regardless of their color. This is assumed to be the result of differences in the production process for crystal and roasted malts. The color of crystal malts is created while the malt is still wet which allows for the formation of more acidic compounds while the color of roasted malts is created after they have already been dried. Though that creates a stronger color it creates less acidity[2] For us brewers this means that grists containing large amounts of roasted malts are likely less acidic than grists containing large amounts of dark crystal malts even though the beers made with roasted malts might be darker in color. | |
|
To get a better understanding what crystal and roasted malts do to the acidity of the grist I randomly generated 210 different grist compositions using the malts I analyzed and the following constraints:
The beer color for all these beers was calculated and mash pH was estimated based on the malt data available. The mash pH was then plotted over the beer color and is shown in Figure 3. It is apparent that the majority of the beers falls into the desired mash pH range if the water has no residual alkalinity (What residual alkalinity is will be explained later in this article). If the waater has moderate residual alkalinity the mash pH will be slightly but not dramatically higher. This is the reason why most average beers can be brewed well with average water. Only at the extremes would be mash pH become a problem. Another observation in this figure is that there is a somewhat linear relationship between beer color and mash pH. The slope of that line is steeper for beers that get all or most of their color from crystal malts and flatter for beers that get their color from roasted malts. This relationship can be used to predict grist acidity from the beer color and the percentage of color that is coming from roasted malt. Eventually this can be used to predict mash pH and such an algorithm is described in Beer color, alkalinity and mash pH and has been implemented in the Kaiser_water_calculator.xls. |
|
Water mineralsThe minerals listed here may either come from the base water, from salts added to the water before dough-in or salts added to the mash. Experiments have shown that the source of the minerals does not make much of a difference in their effect on mash pH. What makes a difference though, is the amount of minerals that are brought into the mash. This amount depends on both mash thickness (how much water per unit of grain) and the mineral concentration in the water. In other words, water in a thin mash will be able to have larger effect on mash pH compared to the same water water in a thick mash since in the latter case there will be less water, and with it less minerals, per grain. This being said, brewers should not conclude that thick mashes provide a practical means of dealing with high alkalinity water just because the alkalinity won’t be able to move the mash pH as much. Any water that is not used in the mash is used during the sparge where it is able to adversely affect pH in both the grain bed and boil kettle. This section focuses only on the water minerals which have an effect on pH. A more complete discussion of brewing water composition can be found in How to read a water report. Bicarbonate and AlkalinityAs mentioned in the beginning, alkalinity is the water’s ability to neutralize acids and the ions involved with that neutralization are bicarbonate (HCO3-) and carbonate (CO32-). More info about carbonate chemistry can be found in About chalk and the carbonate system When brought in contact with the more acidic malt the bicarbonate and carbonate ions absorb hydrogen ions which results in a pH rise. The extent of this pH increase largely depends on the amount of malt, amount of water and the bicarbonate and carbonate concentration in that water. The latter is expressed by the water’s alkalinity. The more bicarbonate and carbonate ions there are per unit of malt the more the pH will rise. When bicarbonate or carbonate neutralizes an hydrogen ion it forms carbon dioxide. While bicarbonate is able to neutralize one hydrogen ion carbonate can neutralize two:
Due to the poor solubility of calcium carbonate and the presence of calcium in most waters, appreciable amounts of carbonate are rarely found in brewing water. As a result the bicarbonate concentration in water can be calculated from the alkalinity of the water and vice versa. Carbonate ions will be present when chalk is added to the water or the mash. The addition of chalk, however, will be discussed later. Calcium and MagnesiumBrewing water also contains calcium and magnesium ions. These ions are able to react with phosphates from the malt to form insoluble phosphate salts which precipitate. At mash pH values between 5 and 6 most of the phosphate is available as HPO42-. The reaction with calcium liberates hydrogen ions which react acidic and lower the pH of the mash [3]:
Magnesium shows a similar but less effective reaction with phosphates which is why it has only half the pH lowering power of calcium. Malt contains about 1 % of phosphate by weight[4] . About 80% of that end up in the wort. This amount is far greater than the calcium or magnesium that is brought in with the brewing liquor which makes calcium and magnesium the limiting factor. As early as 1914 did the German brewing scientist Windish show that water ions have an effect on the mash pH. Kolbach, another German brewing scientist, later showed that it takes about 1.75 calcium ions and twice as many magnesium ions to produce one hydrogen ion. (1.75 calcium ions equal 3.5 calcium equivalents) My own research on this subject reported slightly lower numbers: 1.3-1.5 Calcium ions and 2.4-2.8 magnesium ions[2]. It should be noted that Kolbachs work was primarily targeted at the cast out wort pH and not so much mash pH. There is however a close correlation between the two. Residual AlkalinityThe acidic reaction of calcium and magnesium counteracts the alkaline reaction of the water’s alkalinity, which prompted Kolbach to define the residual alkalinity as the alkalinity that remains after the calcium and magnesium reactions have been considered. Based on his work the following formula for residual alkalinity RA has been established in the brewing world [5] Where:
The residual alkalinity is a property of the brewing water and allows brewers to estimate a water’s effect on the mash pH. If only alkalinity and general hardness (GH) are known the residual alkalinity can be estimated as: This makes the assumption that about 30% of the water hardness comes from magnesium and the remaining 70% come from calcium, which tends to be the average split between calcium and magnesium hardness in typical waters (see Estimating Residual Alkalinity). Water with a residual alkalinity of 0 gives about the same mash pH as distilled water while water with a RA greater than 0 yields a higher mash pH. If the water’s alkalinity is low but its calcium and magnesium levels are high the residual alkalinity can be less than 0. In this case the use of that water will yield a lower mash pH. It should be noted that Kolbach's work focused on the pH of the knock-out wort and not mash pH. Wile there is a close relation his finding that a residual alkalinity change of 10 dH (degrees German hardness, about 171 ppm as CaCO3) causes a 0.3 pH change in pH applies to a 12 Plato cast out wort and not mash pH. The change in mash pH will be less and also depends on mash thickness. Residual Alkalinity illustrated explains the concept of residual alkalinity through a few drawings of ions in the water rather than through chemical formulas alone. AcidsWe may also add acids to the water or mash to lower mash pH below the pH that is established by water and malt alone. This is in particular necessary for lighter beers where the grist itself is not acidic enough to establish the correct pH. Acids donate hydrogen ions which neutralize the alkalinity of the water. Once all the water's alkalinity is consumed the surplus of hydrogen ions counts towards lowering the mash pH. Other factorsThe mash pH can also be affected by the chosen mash schedule. The boiling of mash in decoction mashing, for example, lowers the mash pH. I have seen a pH drop of up to 0.2 pH units during decoction mashing. This decrease of mash pH might be attributed to the enhanced precipitation of calcium and magnesium phosphates [3]. A rest between 30 and 40 C (90-110 F), known as acid rest, promotes phytase activity which releases more phosphates into the mash. While this is said to acidify the mash I have not observed a significant mash pH decrease though the use of this rest. | |
Strategies for affecting mash pHWhen the expected or tested mash pH is outside the desired range of 5.3-5.5 the brewer may choose to change that pH. This can be done by:
|
|
Changing the gristThere was a time when brewers didn't know about water chemistry. They used the available local water and noticed that some beers turned out better than others. This led to the development of local beer styles. But these days we brewers don't want our water to dictate what beer styles we should brew. As a result changing the grist is rarely seen as a viable option. The only case where a grist change is practical is the use of acidulated malt or Sauermalz. Acidulated malt is Pilsner malt that has been sprayed with lactic acid before it is dried again. The final lactic acid content of this malt is about 3% by weight. Each 1% of acidulated malt in the grist lowers the mash pH by ~0.1 pH units. More than 4-5% should not be used in order to prevent excessive lactic acid amounts that may be noticeable in the final beer taste. The use of Sauermalz is an elegant way of complying with the German purity law (Reinheitsgebot) since its lactic acid has been produced by lactic acid bacteria that are naturally occurring on the surface of the malt. Because of that the mash pH can be corrected and the beer can still be brewed with just water, malt, hops and yeast. Some German brewers believe that if this loophole would not have been found the German purity law would have needed to be amended since the benefits to beer quality attained from being able to acidify the mash are substantial. More info about the use of lactic acid can be found later in Adding acids Changing the waterBefore the water can be changed we need to know more about the mineral content of the water. How to read a water report explains in detail the various minerals found in drinking water. It also explains what to look for in a water report. When the water is changed to correct the mash pH it’s residual alkalinity is changed to match the needs of the grist composition used in the recipe. The desired residual alkalinity range can be estimated from the beer’s color. The darker the beer the more residual alkalinity will be needed to counteract the acidity of the grist. The residual alkalinity’s effect on pH also depends on mash thickness. The thinner the mash the more pronounced the effect of the water’s alkalinity will be. At a mash thickness of 2 l/kg (0.95 qt/lb) it takes a residual alkalinity change of about 130 ppm as CaCO3 (2.6 mEq/l) to change the mash pH by 0.1 pH units while it takes a residual alkalinity change of just 75 ppm as CaCO3 (1.5 mEq/l) to achieve the same pH change at a mash thickness of 4 l/kg (1.9 qt/lb)[2]. Raising residual alkalinity (raises mash pH)If the water’s residual alkalinity is too low the addition of alkaline salts like calcium carbonate (chalk, CaCO3) or sodium bicarbonate (baking soda, NaHCO3) or even strong bases like calcium hydroxide (Ca(OH)2) raises its residual alkalinity. If calcium carbonate is used it will not dissolve in the water unless CO2 is added. Though there are ways to dissolve calcium carbonate many brewers simply add it to the brewing water without dissolving it. In experiments I found that dissolved chalk is not only twice as effective in raising the water’s residual alkalinity, undissolved chalk is also not able to raise the mash pH by more than 0.2 pH units. In other words the addition of more than 500 mg/l undissolved calcium carbonate, which is equivalent to a residual alkalinity of about 200 ppm as CaCO3, had little or no effect on mash pH[2]. As shown in Beer color, alkalinity and mash pH water with a residual alkalinity of more than 200 ppm as CaCO3 is rarely needed, even for the darkest beers. A.J. deLange, an expert in everything brewing water, believes that while the mash pH is low enough to dissolve chalk, the actual rate of dissolving chalk at this pH happens slowly which reduces it's effectiveness as a means of raisinbg mash pH[6]. Despite its shortcomings it still remains the most popular salt addition for raising mash pH. In addition to carbonate chalk also adds calcium to the mash which counteracts some of the pH raising power of the carbonate. Baking soda, however, is more soluble than chalk and does not show the somewhat unpredictable mash pH behavior that comes with chalk. The drawbacks of adding baking soda is the increase of the water’s sodium content and the lack of calcium which has a number of positive effects on beer quality. Another substance that can be used to increase the alkalinity of the brewing water and thus raise the mash pH is calcium hydroxide (pickling lime, slaked lime, CaOH). It dissolves in water more readily than chalk and doesn't show the limits that undissolved chalk has while it also adds calcium to the mash. The only drawback is that it is a caustic substance and needs to be handled with care. It is best added to the mash after dough-in. Lowering residual alkalinity (lowers mash pH)In most cases the residual alkalinity of the water is too high for the desired beer color which causes the mash pH to be too high as well. In these cases the water’s residual alkalinity needs to be reduced. For that we brewers have a number of options:
DilutionThe idea of dilution is simple: reduce the bicarbonate concentration, and with it the alkalinity, by mixing the water with water that contains only little bicarbonate. The water generally used for dilution is distilled or very low mineral water like reverse osmosis water. When using such water for dilution the residual alkalinity of the diluted water is Where:
Since dilution with low mineral water lowers the concentration of all minerals it may be necessary to supplement the resulting water with calcium ions to get their concentration back into the desired range of 50-150 ppm. This should be done with calcium chloride or calcium sulfate (gypsum) in order to avoid adding bicarbonate which would negate the alkalinity lowering effect of dilution. The added benefit of supplementing calcium is an additional residual alkalinity reduction through the acidic reaction between calcium and malt phosphates. Adding CalciumAs already discussed in [[#Calcium and Magnesium]|Calcium and Magnesium] calcium and magnesium salts are able to lower mash pH trough a reaction with phosphates brought in by the malt. This is of particular interest for beer styles that benefit from water with high permanent hardness. In permanently hard water the anions (negatively charged ions) balancing the calcium and magnesium cations are chloride and sulfate which have no effect on the mash pH. This is not true for temporary hard waters where the balancing anion is bicarbonate. Examples for these styles of beer are English Ales and Dortmunder Export. A mash water calcium content of 150 mg/l and little or no alkalinity can yield a mash pH drop of 0.1 – 0.2 pH units depending on mash thickness. Due to its lower effectiveness with respect to changing the mash pH and its lower desired concentration in brewing water, magnesium salts are generally not used to affect mash pH in any meaningful way. Adding acidsThe water’s bicarbonate content, and with it the alkalinity and residual alkalinity, can also be lowered or completely removed through the addition of organic or inorganic acids. The hydrogen ions released from these acids react with the bicarbonate to form carbon dioxide and water:
When acids are used to reduce the alkalinity bicarbonate is replaced with the anion provided by the acid. As a result, excessive use of acids, which may seem necessary with very alkaline waters, can lead to an excess of these ions and an adverse effect on taste. To prevent this water alkalinity may first need to be reduced with means that lower the water’s mineral contents like dilution or alkalinity precipitation, for example. While many acids can be used to accomplish this task only a few have found practical use for water treatment in brewing. Those acids are:
If more acid than what is needed to neutralize all alkalinity is added, the alkalinity of the water becomes negative. The acid that was not used for neutralizing alkalinity will contribute to lowering the mash pH once the malt is added to the water. In order to get the mash pH within the desired 5.3-5.6 range when brewing very pale beers, Pilsners for example, it is generally necessary to use an acid regardless how low the residual alkalinity of the brewing water is. This is because even calcium contents on the high end (150 ppm) and an alkalinity of 0 yields a residual alkalinity of only -70 ppm as CaCO3. This residual alkalinity can only lower the mash pH by ~0.2 units when a 4 l/kg (2 qt/lb) mash thickness is used and even less for thicker mashes. With a distilled water mash pH for pilsner malt that tends to be between 5.7 and 5.8 the resulting mash pH without acid additions would only be in the 5.5 to 5.6 range. 2% Sauermalz will get it into the for lighter beers more preferable 5.3-5.4 range. Precipitation of calcium carbonateSince the addition of acid does not change the total amount of minerals it may not be suited for the treatment of waters that contain high concentrations of minerals. One method suited for these waters is the precipitation of calcium carbonate through either boiling or the addition of slaked lime. The calcium, and to some extent magnesium, and alkalinity removed through these methods are called temporary hardness. Temporary hardness is the calcium and magnesium ions that can be matched up with bicarbonate ions from the water. In fact, temporary hardness is called temporary because it can be removed through boiling. The remaining calcium and magnesium, which has only sulfate and chloride to pair up with is called permanent hardness. When water is boiled CO2 escapes. This raises the water pH and leads to the creation of more carbonate from the existing bicarbonate. In the presence of calcium the carbonate forms calcium carbonate (a.k.a chalk, CaCO3) which is poorly soluble and creates a white precipitate which settles out. If you have water with a high temporary hardness (lots of bicarbonate calcium and magnesium) you may have already noticed this when you cook with your water.
This method is able to lower the alkalinity to about 50 - 65 ppm as CaCO3 [8]. The amount of alkalinity that can be removed is the difference between the current alkalinity and the lowest limit (50 - 65 ppm as CaCO3) or the calcium hardness, whichever is lower. Sufficient calcium content (i.e. calcium hardness) is needed since the precipitation of 1 ppm alkalinity as CaCO3 also precipitates 1 ppm calcium hardness as CaCO3 or 0.4 mg/l calcium. As a result the water may become calcium deficient. The final calcium level can be calculated with this formula: Where
If the resulting Calcium concentration is below the recommended minimum of 50 mg/l gypsum or calcium chloride should be added to boost the initial calcium content before the water is boiled. In addition to that the addition of a small amount of chalk facilitates this precipitation by providing nucleation sites for the precipitating chalk [9]. Water exposed to atmospheric CO2 pressure can only keep about 47 mg/l calcium carbonate (chalk) in solution[10]. This amounts to an alkalinity of about 50 ppm as CaCO3 which is less than the lowest possible alkalinity level cited above and as a result allowing the water to stand for an extended time after it has been boiled does not risk re-dissolving the precipitated chalk though its uptake of atmospheric CO2. If a GH&KH test kit is available the final hardness and alkalinity achieved through boiling can be checked and used for the estimation of the mash pH (see At home water testing). While the aforementioned process is simple, the need to boil a large amount of water represents a great deal of wasted energy. The excess CO2 can also be removed from the water through intensive aeration but that process takes a long time. In fact, surface water tends to have low temporary hardness because due to the lower CO2 content in air the water’s CO2 content is much lower than that possible in ground water and as a result this water cannot hold as much calcium carbonate as ground water. A more practical approach, that is used by many breweries, is water treatment with slaked lime. The slaked lime is able to absorb the water’s CO<sub<2</sub> and raise the water pH to transform bicarbonate into carbonate:
How to conduct this water treatment and calculate the amount of lime needed has been described in detail in Alkalinity reduction with slaked lime and will not be discussed further. Both boiling and slaked lime treatment will only remove bicarbonate and calcium (to some extend magnesium as well) but cannot remove sodium, chloride or sulfate. Those minerals do not affect pH but if a lower concentration is desired the only practical method the home brewer has for their removal is dilution with low mineral water or building brewing water from scratch. Building brewing water from scratchUsing low mineral water like distilled or reverse osmosis water and adding back measured amounts of various salts is becoming increasingly popular among home brewers. One of the likely reasons is that reverse osmosis (commonly abbreviated as RO) units have become reasonably affordable. Many grocery stores also sell RO filtered water for as low as 25 cents for the gallon. Another factor is that this process provides ultimate control over the brewing water composition. While being enthusiastically embraced by home brewers it is not as economical for larger brewers since the production of 1 liter of reverse osmosis water may require as much as 6 liters raw water though the efficiency of these units largely depends on the mineral content of the raw water and the design of the unit. Reverse osmosis works by pushing water through a filter membrane that has openings large enough for water but not for mineral ions. The result is lower mineral water, the product water, on one side and higher mineral water, the waste water, on the other side. The process is slow and RO units are generally equipped with storage tanks that deliver water when needed. For residential "under counter" units these tanks generally hold only 1.5 - 1.7 gallons which is too little to supply the water needed in brewing. An upgrade to a 7 gallon or even larger tank is recommended. The mineral content of the water should regularly be tested with a TDS (Total Dissolves Solids) meter. These meters measure the electric resistance of the water to determine its mineral contend and tend to be included when a RO unit is purchased. An increase in the RO water's mineral content means that it is time to replace the membrane. I have been using my RO unit for about 4 years now and have not had the need to replace the membrane. RO water is not completely free of minerals. About 5 - 10% of the initial mineral content remains but the resulting water is low enough in minerals that it can be treated as being free of minerals. If so desired you may also send a sample of the RO water to a water analysis lab to get a base water profile for your brewing water additions. When building water from scratch brewers should have the following salts on hand:
Baking soda can be found in the baking isle and Epsom salt can be found in the health section of grocery stores. The other salts are best purchased at a home brewing store. A digital scale for precisely measuring grams of salts is also very helpful. I have had very good success with jewelry scales (100-200g capacity, 0.01g resolution) that can be found on e-bay for ~$20. Water modification spreadsheetCalculating and predicting the results of water modification can be complicated due to the chemistry involved. To simplify these calculations a water calculation spread sheet has been developed. This spreadsheet supports:
Designing and modifying brewing waterUsing a water spreadsheet and determining the necessary water modifications is one of the more daunting aspects or water chemistry for brewers that want to start taking charge of their water. There are just too many variables that can be changed. Here are some guidelines that can help:
Keep in mind that a salt always add both an anions (-) and a cations (+). You cannot add calcium without also adding chloride, sulfate for alkalinity, for example. This means some tweaking is necessary to get to the desired levels. Various water recipes lists a few water recipes that have worked well for me in the past. Historic and current water profiles of major brewing cities may also be used as a guideline for a particular beer. But it is important to understand that these waters may have undergone water treatment before being used to brew a style that originated from that region. If the historic water profile fits the beer that you are brewing, i.e. the mash pH is in an acceptable range, you may want to target this water since brewers may most likely have been using this water for their beers. A classic example for this is the water profile for Munich. While this water is great for brewing a Munich Dunkel or a Doppelbock it is the wrong water for brewing a Munich Helles. This largely Pilsber malt based beer requires water with low residual alkalinity. To get to the desired mash pH Munich brewers likely precipitate alkalinity from the water using lime and in addition to that use sour fermented wort (Sauergut) to correct the mash pH even lower. The Paulaner brewery, for example, doesn't even have to decarbonate their water. They are getting very soft (~ 2 dH, 40 ppm as CaCO3) brewing water from a deep well on their property. | |
Salt and acid additions to the mashMash pH and later wort pH can also be adjusted on the fly. This is generally done by adding either salts or acids to bring the pH back into the desired range after it has been tested and was found to be too high or too low. Since the malt is the dominating pH buffer the calculation of the necessary acid or salts are best based on the grist weight while mash thickness (i.e. the amount of mash water used) matters little. All salts and acids that can be added to the water can also be added to the mash. If salts are added only to the mash their effective concentration in the beer will be lower since the additional water used for sparging will dilute the added minerals in the kettle. While it is practical to treat water and grist such that they achieve the desired mash pH at dough-in, many brewers find it easier to measure the pH of a mash sample 5-10 min after dough-in and base the addition of salts and acids based on how far away this pH is from the targeted pH. This works particularly well for brewers who rarely repeat recipes and thus have less past data that could be used to estimate the necessary water and mash treatments. While there doesn’t appear to be any difference between treating the water and treating the mash with respect to the pH the mash will settle at, there can be a slight difference in expression of enzymatic activity. In particular in single infusion mashing where the bulk of the fermentable sugars is created by beta-amylase early during the mash before large amounts of that enzyme fall victim to the mash temperature. This enzyme has a pH optimum between 5.3 - 5.6 [7] and if the pH of the mash is sufficiently far off this optimum during the first 10-20 min the amount of fermentable sugars produced and with it the fermentablility of the created wort may suffer. Table 3 contains a summary of the possible pH treatments that can be made to the mash or to the strike water. |
|
References
|